Crystal lattice- Definition, Types, examples, lattice point
The crystal lattice is a fundamental concept in chemistry that helps in understanding the arrangement of atoms, ions, or molecules in a crystalline solid. A crystal lattice refers to a repeating three-dimensional pattern of particles that extends throughout the entire crystal.
This article will explain the concept of the crystal lattice in detail, along with examples and the concept of a lattice point.
What is a crystal lattice?
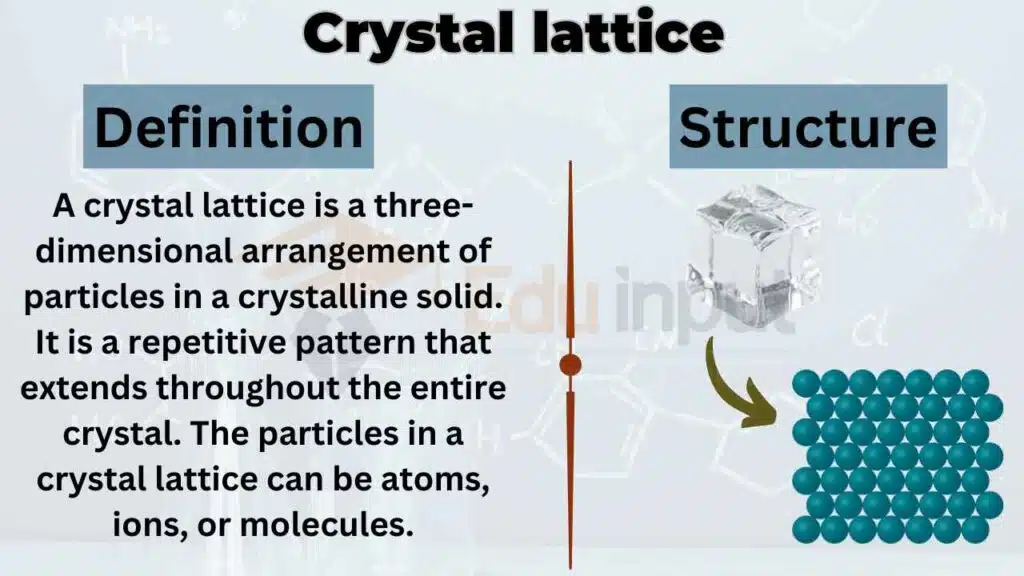
A crystal lattice is a three-dimensional arrangement of particles in a crystalline solid. It is a repetitive pattern that extends throughout the entire crystal. The particles in a crystal lattice can be atoms, ions, or molecules.
The arrangement of these particles is such that they form a pattern that repeats in all three dimensions. This pattern is responsible for the characteristic shape and structure of a crystal.
Types of crystal lattices
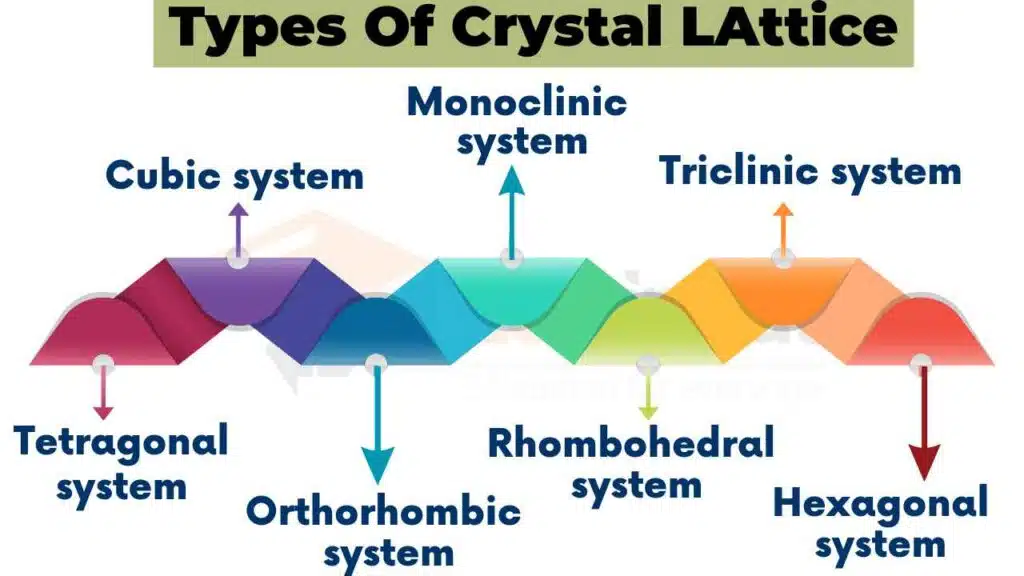
There are 14 types of crystal lattices, also known as Bravais lattices. These lattices are named after Auguste Bravais, who discovered them. These lattices can be classified into seven different systems based on their symmetry. These systems are:
1. Cubic (or Isometric) System
- Shape: Perfect cubes.
- Features: Equal axes that are perpendicular to each other.
- Examples: Salt (sodium chloride), diamonds.
2. Tetragonal System
- Shape: Similar to cubic but with a longer or shorter axis.
- Features: Two axes are of equal length, and the third is different; all are perpendicular.
- Examples: Zircon, wulfenite.
3. Orthorhombic System
- Shape: Stretched or compressed cubes.
- Features: Three different axes, all perpendicular to each other.
- Examples: Topaz, olivine.
4. Hexagonal System
- Shape: Six-sided prisms.
- Features: Four axes; three are of equal length and lie in the same plane, and the fourth is perpendicular.
- Examples: Quartz, beryl.
5. Trigonal System (or Rhombohedral)
- Shape: Similar to hexagonal but with a twist.
- Features: Axes of equal length but not at right angles.
- Examples: Calcite, cinnabar.
6. Monoclinic System
- Shape: Inclined prisms.
- Features: Two axes are perpendicular, and the third is at an angle.
- Examples: Gypsum, and jadeite.
7. Triclinic System
- Shape: Uneven, asymmetric shapes.
- Features: All axes are of different lengths and none are perpendicular.
- Examples: Turquoise, kyanite.
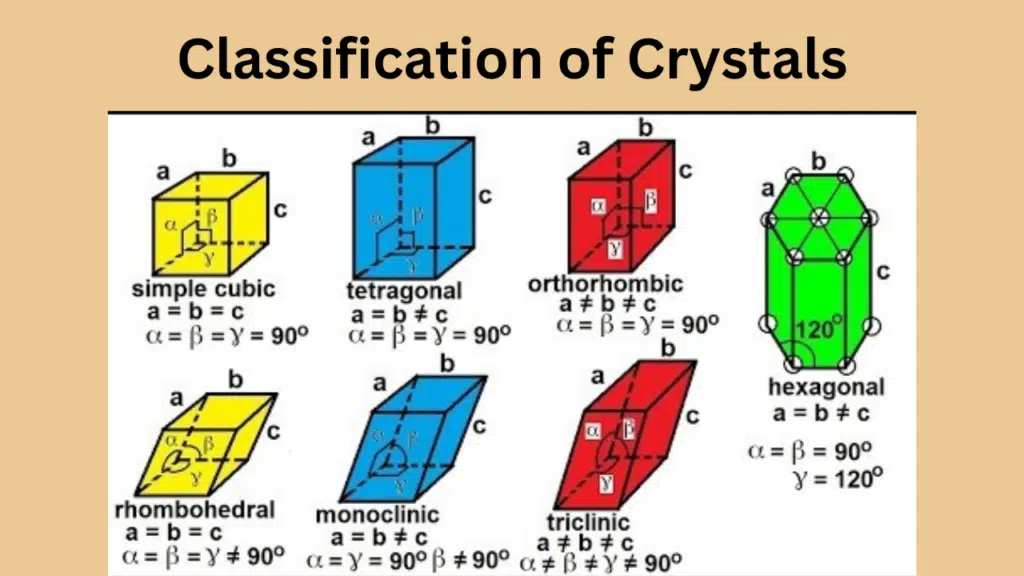
| Sr. | Crystal System | Axes | Examples |
| 1 | Cubic | a = b = c | Fe, Cu, Ag, Au, NaCl, NaBr, Diamond |
| 2 | Tetragonal | a = b ≠ c | Sn, SnO2, MnO2, NH4Br |
| 3 | Orthorhombic | a ≠ b ≠ c | Iodine, Rhombic Sulphur, BaSO4, K2SO4 |
| 4 | Monoclinic | a ≠ b ≠ c | Sugar, Sulphur, Borax, Na2SO4.10H2O |
| 5 | Hexagonal | a = b ≠ c | Graphite, ZnO, CdS, Ice, Zn, Cd |
| 6 | Rhombohedral or Trigonal | a = b = c | Bi, Al2O3, NaNO3, KNO3 |
| 7 | Triclinic | a ≠ b ≠ c | HBO2, K2Cr2O7, CuSO4.5H2O |
Why does Crystal Classification Matter?
The classification of crystals is not just for academic interest. It has practical implications in various fields:
- Materials Science: Understanding crystal systems helps in designing and synthesizing new materials.
- Geology: Helps in identifying minerals and understanding the earth’s composition.
- Technology: Used in creating electronic components, lasers, and optical devices.
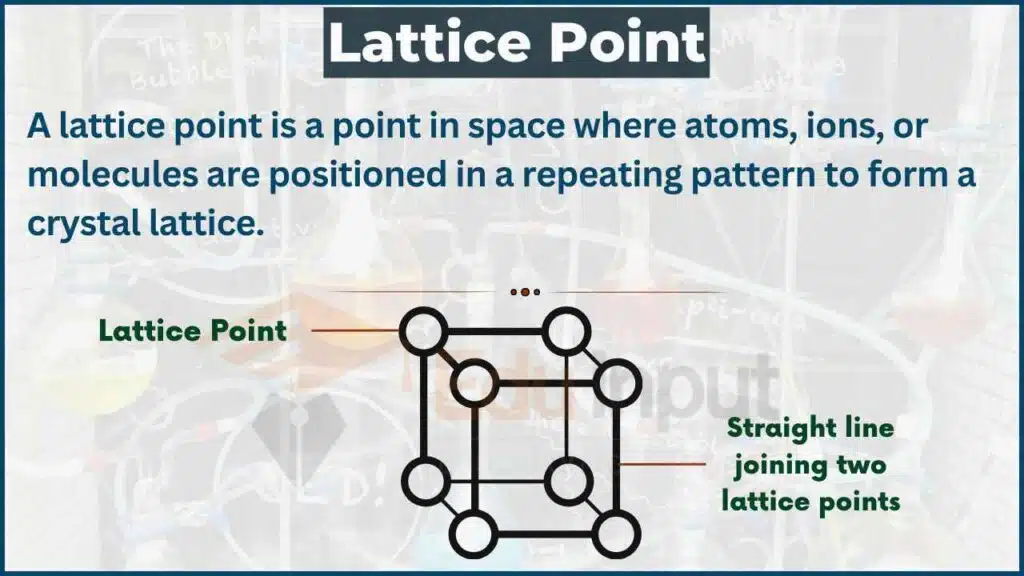
What is a lattice point?
A lattice point is a point in a crystal lattice where the particles are located. These points are the intersections of the lattice lines, which represent the paths between the particles. The position of the lattice points determines the crystal structure.
Examples of crystal lattices
There are different types of crystal lattices, which are given below:
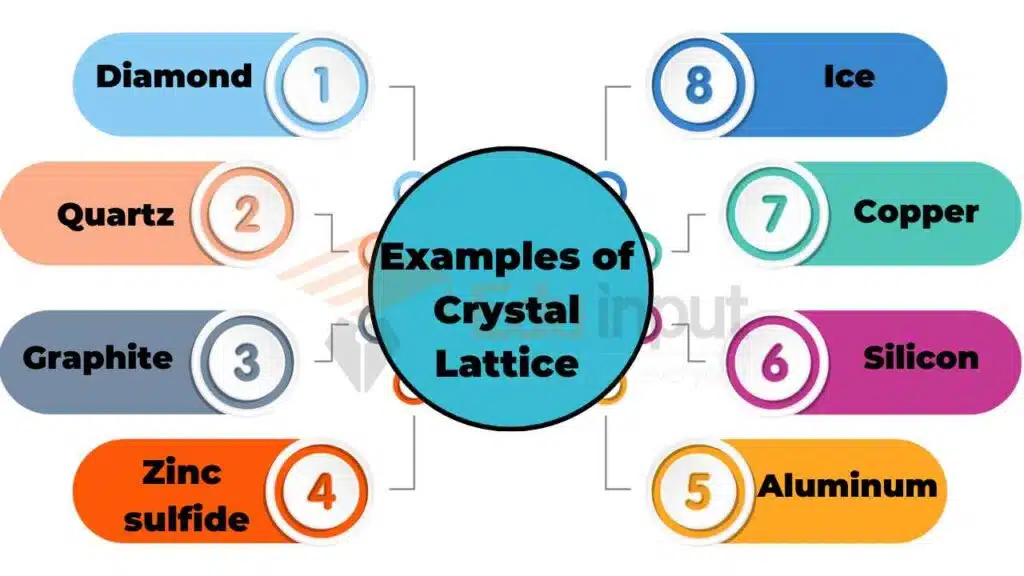
- Sodium chloride: Sodium chloride, also known as table salt, has a face-centered cubic lattice structure.
- Diamond: Diamond has a cubic lattice structure.
- Quartz: Quartz has a hexagonal lattice structure.
- Graphite: Graphite has a hexagonal lattice structure.
- Zinc sulfide: Zinc sulfide has a zincblende lattice structure.
- Ice: Ice has a hexagonal lattice structure.
- Copper: Copper has a face-centered cubic lattice structure.
- Silicon: Silicon has a diamond lattice structure.
- Potassium chloride: Potassium chloride has a face-centered cubic lattice structure.
- Aluminum: Aluminum has a face-centered cubic lattice structure.
In conclusion, a crystal lattice is a fundamental concept in chemistry that describes the repeating pattern of particles in a crystalline solid. There are 14 types of crystal lattices, classified into seven different systems based on their symmetry. Lattice points are the points in a crystal lattice where the particles are located, and the position of these points determines the crystal structure. Examples of crystal lattices include sodium chloride, diamond, quartz, graphite, zinc sulfide, ice, copper, silicon, potassium chloride, and aluminum.





Leave a Reply