Difference between pH and pOH
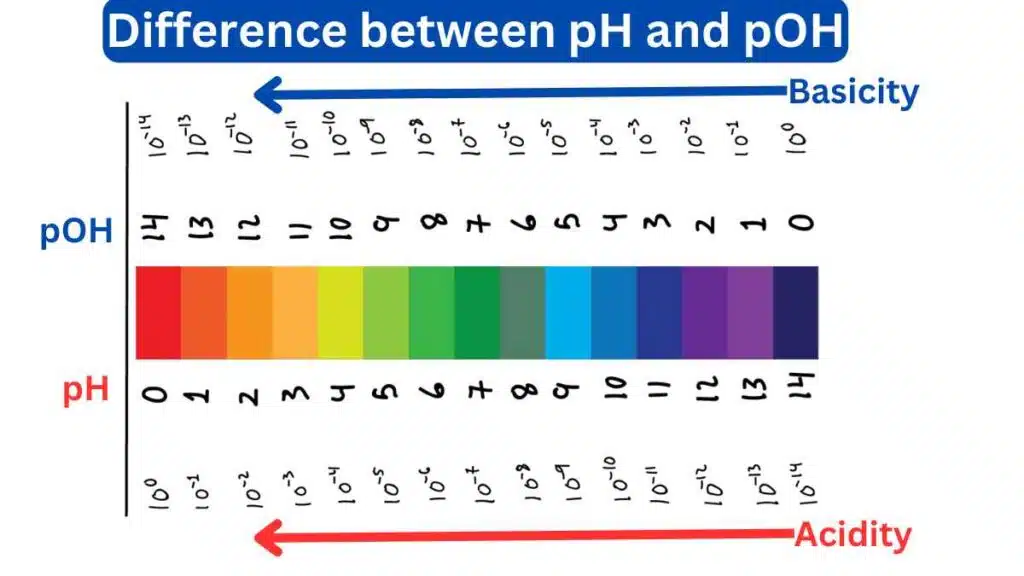
The main difference between pH and pOH is that pH measures the concentration of hydrogen ions (H+) in the solution, with higher values indicating increasing acidity. pOH, on the other hand, measures the concentration of hydroxide ions (OH–), with higher values indicating increasing alkalinity.
What is pH?
pH is a measure of the acidity or alkalinity of a solution. It indicates the concentration of hydrogen ions (H+) present in the solution. Acidic solutions have a higher concentration of H+ ions and lower pH values, while alkaline or basic solutions have lower H+ concentrations and higher pH values.
The pH scale ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most alkaline or basic. The pH of a solution is calculated as the negative logarithm of the H+ ion concentration.
What is pOH?
pOH is a measure of the hydroxide ion (OH–) concentration in a solution. It is related to pH in that as the pH of a solution goes up, the pOH goes down. pOH indicates the basicity or alkalinity of a solution, with lower pOH values corresponding to higher OH– concentrations and more basic solutions.
The pOH scale also ranges from 0 to 14, with 14 being the most basic or alkaline. The pOH of a solution is calculated as the negative logarithm of the OH– ion concentration.
Difference Between pH and pOH
There are some key differences between pH and pOH.
| Characteristic | pH | pOH |
|---|---|---|
| Definition | pH measures the acidity or alkalinity of a solution based on the concentration of hydrogen ions (H⁺). | pOH measures the alkalinity or acidity of a solution based on the concentration of hydroxide ions (OH⁻). |
| Formula | pH = -log[H⁺] | pOH = -log[OH⁻] |
| Range | Typically, pH values range from 0 to 14. | Typically, pOH values range from 0 to 14. |
| Neutral Value | pH 7 is considered neutral (pure water). | pOH 7 is considered neutral (pure water). |
| Acidic Solutions | Lower pH values indicate more acidic solutions, with pH 0 being the most acidic. | Higher pOH values indicate more acidic solutions, with pOH 14 being the most acidic. |
| Alkaline Solutions | Higher pH values indicate more alkaline solutions, with pH 14 being the most alkaline. | Lower pOH values indicate more alkaline solutions, with pOH 0 being the most alkaline. |
| Relationship | pH + pOH = 14 (at 25°C) | pH + pOH = 14 (at 25°C) |
| Example | A solution with pH 3 is acidic. | A solution with pOH 11 is alkaline. |



Leave a Reply