Energies of bond formation | Energetics of Hydrogen bond formation
During the process of chemical bonding when a chemical bond is formed, energy is decreased. Various types of forces operate during bond formation. to understand this concept let us take an example of hydrogen molecule formation.
Formation of H2 Molecule
Consider the example of two H atoms approaching each other. Both the forces of attraction and the force of repulsion act at the same time. As the atoms come close to each other, Potential energy decreases. The forces of attraction try to bring the atoms close to each other.
The forces of repulsion try to push the atoms away from each other. Hence, potential energy increases. It has been studied that the magnitude of potential energy is less for attractive forces than for the forces of repulsion. That is why potential energy decreases as hydrogen atoms come close to each other.
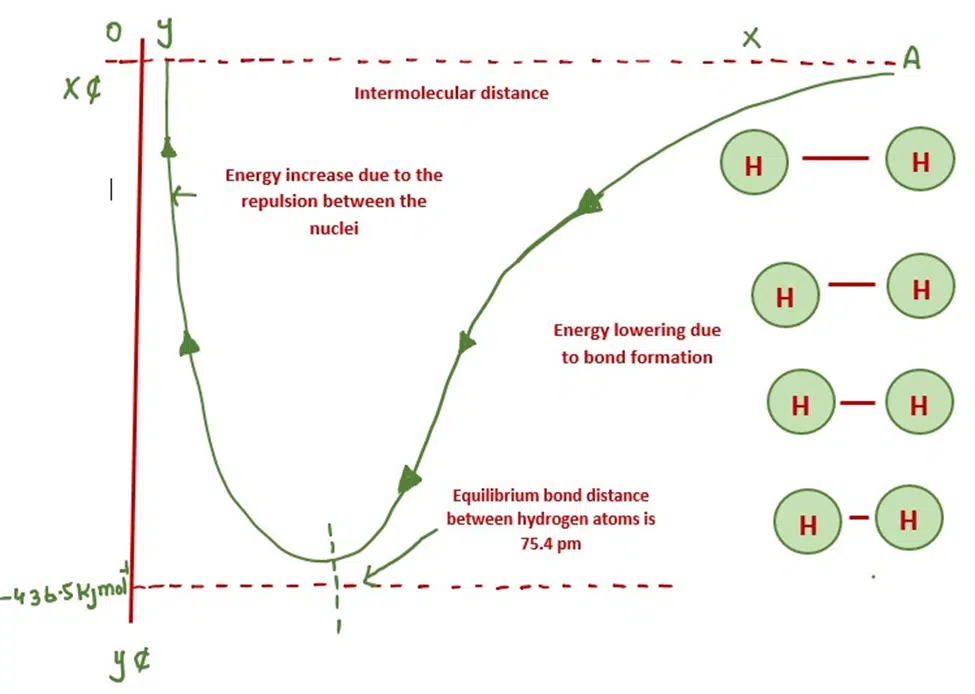
Eventually, both the atoms come closer to a distance of 75.4pm. At this distance, the forces of attraction are greater than the repulsive forces. When the distance between the two hydrogen atoms decreases beyond 75.4pm. The repulsive forces become dominant and the system becomes unstable.
The distance of 74.5pm is called bond distance or compromise distance of the lowest energy. The amount of energy evolved id 436.5 kJmol-1. It is called bond formation energy.
In order to break the bond, the same amount of energy is to be provided. If the repulsion forces are greater as in the case of ‘He’ atoms, the energy of the system increases and so there will be no bond formation.
Hope you understand this example, if you have any questions about this topic let us know in the comment section. We will answer your questions.






Leave a Reply