Energy Band-Definition, And Energy Band Theory
According to the concept of an energy band, the atoms in a crystal stone can be more closely spaced apart and many electrons can interact with one another. Their energy levels can alter in response to variations in the electron energy levels within their shell.
Energy Band
Molecules are arranged differently in solids, liquids, and gases. They have gathered closely together to form solids, which has caused the electrons in the molecule’s atoms to move into the orbitals of their neighbors.
In liquids, the molecular organization is moderate, but it is not close to gases. As a result, the electron orbitals partially cover as the atoms get close to one another.
Instead of single energy levels, the levels of energy bands are produced by the fusion of atoms inside materials. A group of closely spaced energy levels is referred to as an energy band.
The main feature of the energy band is that electron energy levels in electronics remain constant throughout a broad frequency range. As a result, an atom’s energy level will differ in its valence and conduction bands.
Energy Band Theory
According to Bohr’s hypothesis, each of an atom’s shells has a specific amount of energy at various levels. The interaction of electrons between the outermost and innermost shells is explained by the energy band theory. There are three distinct energy bands according to the energy band theory:
- Valence band
- Forbidden energy gap
- Conduction band
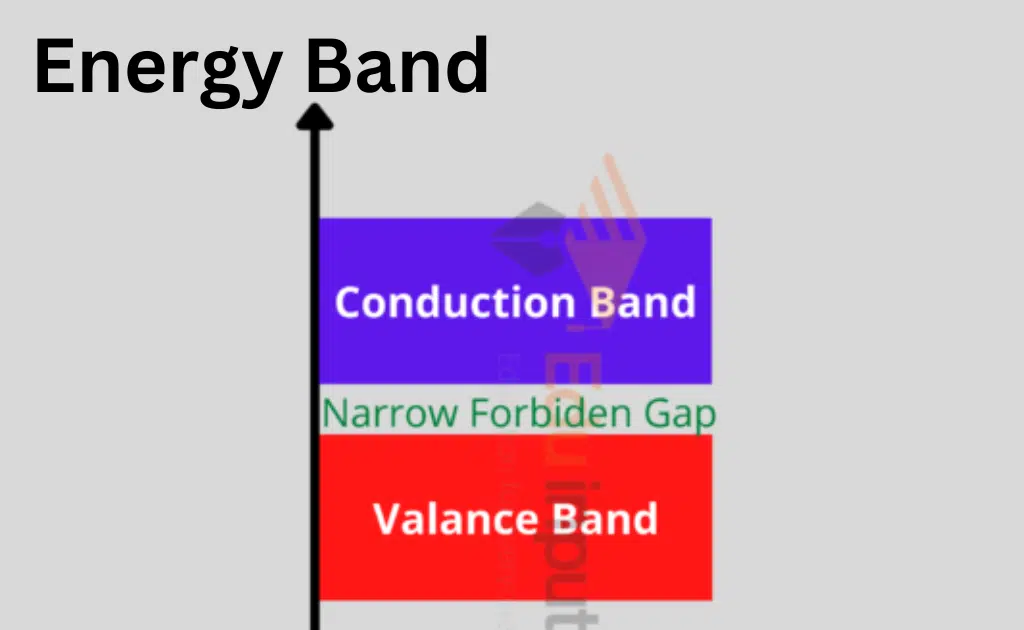
Valence Band
The energy of an electron in the inner shell is higher than that of an electron in the outer shell, although electrons move at constant energy levels within atoms. The electrons that are located inside the outer shell are known as value electrons.
The valence band is composed of several energy levels that make up these electrons. The most energy is occupied in this band.
Forbidden Energy Gap
The prohibited gap is the space between the valence band and the conduction band. The forbidden gap, as its name indicates, has no energy, and no electrons remain in this band.
The valence band electrons are securely bound or firmly bonded to the nucleus if the forbidden energy gap is higher. To fill the prohibited energy gap, we need some external energy.
Conduction Band
A few of these valence electrons can escape the outermost orbit even at ambient temperature and become free electrons because they are not securely bound to the nucleus. The unbound electrons are referred to as conduction electrons because they may move current through conductors.
The band with the lowest occupied energy levels is the conduction band, which also includes conduction electrons.
Related FAQs
What is the energy band of a semiconductor?
According to the definition of an energy band, more electrons will interact with one another and more atoms within a crystal stone may be closer to one another. Changes in the energy levels of electrons within their shell may be the cause of those changes.
What is the energy band in a solid?
Each electron has a varied energy level within a solid crystal due to slightly varying patterns of the surrounding charges. The Energy Bands, a continuous energy fluctuation made up of these electron energy levels, are so named.
What is the energy band principle?
The quantum state that an electron assumes inside a solid metal is described by the band theory of solids. There are numerous distinct energy levels present in every molecule. Band theory does a good job of explaining how electrons behave inside molecules.
How energy band is formed?
Atoms tend to move into the orbits of the atoms that are close to them because of the way that molecules are arranged in solids. Therefore, when atoms combine, electron orbits overlap. By intermixing atoms in the solid state, several bands of energy levels are formed. These energy levels are called Energy Bands.





Leave a Reply