Helium-Discovery, Properties, And Applications
Helium is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, and almost completely inert gas.
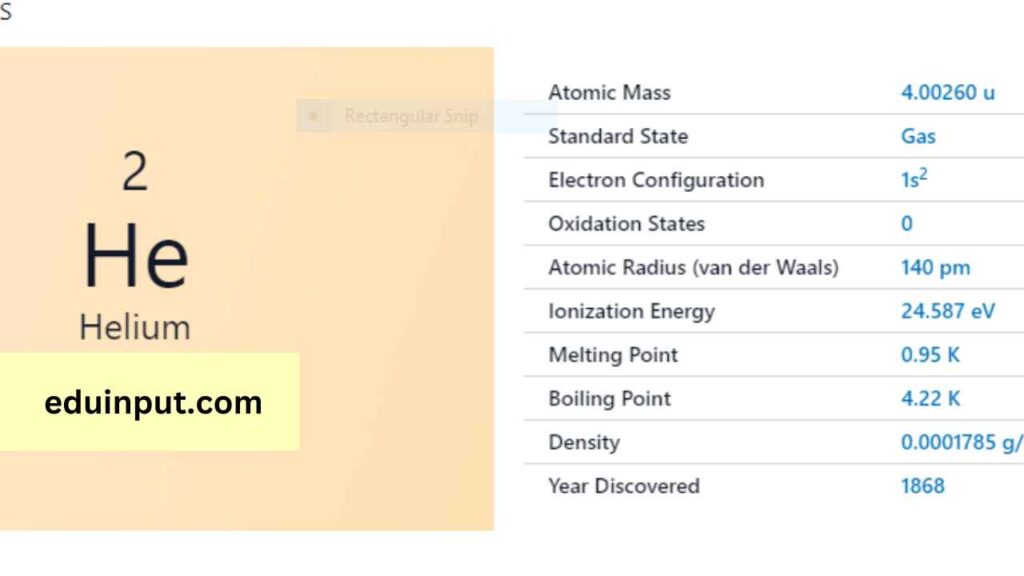
| Property | Value |
| Name | Helium |
| Symbol | He |
| Atomic number | 2 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Gas at 298 K |
| Appearance | Colourless |
| Classification | Non-metallic |
| Group in periodic table | 18 |
| Period in the periodic table | Noble gas |
| Block in the periodic table | 1 |
| Block in periodic table | p |
| Shell structure | 2 |
| CAS Registry | 7440-59-7 |
Discovery
Helium was first detected in 1868 by French astronomer Jules Janssen and British astronomer Joseph Norman Lockyer during a solar eclipse. It was named after the Greek god of the Sun, Helios.
Physical Properties
Helium is the second lightest element and is a gas at room temperature. It has the lowest boiling point of all the elements and cannot be solidified by cooling. Helium has two protons and two neutrons in its nucleus and two electrons in its outer shell.
Chemical Properties
Helium is classified as a noble gas, which means that it is almost completely inert and does not easily react with other elements or compounds. It has the highest ionization energy of any element, which means that it requires a lot of energy to remove an electron from a helium atom.
Facts
- Helium is the second most abundant element in the universe, after hydrogen.
- Helium is used to cool magnets in MRI machines and other scientific equipment.
- Helium is lighter than air and is used in balloons and airships.
- Helium is created by the process of nuclear fusion in stars.
- Helium has the lowest boiling point of any element and cannot be solidified by cooling.
Applications
- Helium is used as a cooling agent in MRI machines, particle accelerators, and other scientific equipment.
- Helium is used to pressurize and test rocket engines.
- Helium is used in welding, as a protective gas to prevent contamination of the weld area.
- Helium is used in airships and balloons due to its low density.
- Helium is used as a lifting gas for blimps and balloons.






Leave a Reply