Difference Between Intrinsic And Extrinsic Semiconductor
The main difference between intrinsic and extrinsic semiconductor is that a pure semiconductor is intrinsic and an impure semiconductor is known as an extrinsic semiconductor. A semiconductor is a material that is not a good conductor and not a bad insulator.
Intrinsic Semiconductor
A semiconductor in its pure form is called an intrinsic semiconductor.
The electrical properties of the semiconductor purely depend upon the purity of the semiconductor
Example: Silicon and Germanium are pure semiconductors
What is Doping?
The process of adding selected impurities in the pure semiconductor is called doping.
The impurity atoms are added in 1 to 106 ratios.
Extrinsic Semiconductor
The impure semiconductor in which an impurity is added is called an extrinsic semiconductor.
Electronic Structure of Extrinsic Semiconductor
Since the semiconductors belong to the fourth group of the periodic table. Therefore they have four electrons in their valence shell.
Each atom in the semiconductor material is regularly arranged and forms four covalent bonds with its neighbors.
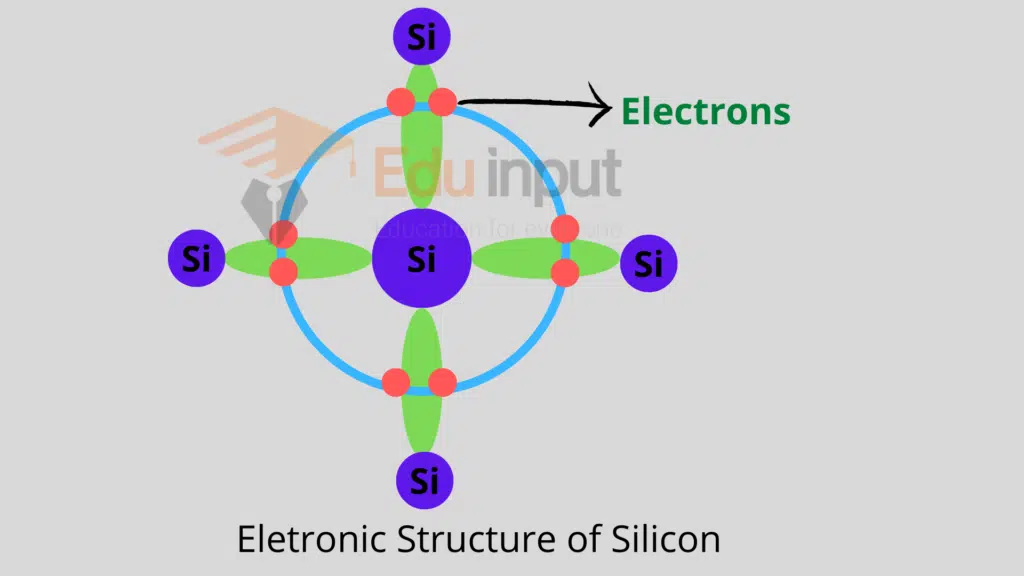
This effectively allocates eight electrons to the outermost orbit of each atom which is a stable state. Due to these covalent bonds, the electrons are bound in their respective shells.
N-Type Semiconductor
When a silicon material is doped with a pentavalent impurity i.e. atom from the fifth group like arsenic, antimony or phosphorus, etc.
Four electrons of the impurity atom make four covalent bonds with the silicon atoms and the fifth electron remain un-bonded.
This becomes the free electron in the crystal. This type of doped or extrinsic semiconductor is called an N-type semiconductor.
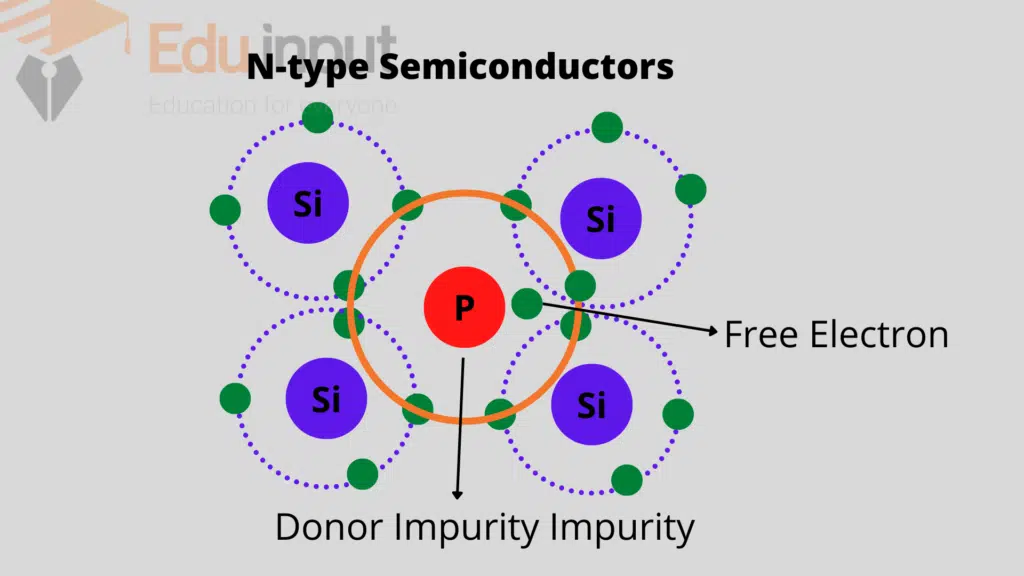
The impurity atom is called the donor atom because it donates a free electron. This electron is thermally excited into the conduction band
P-Type Semiconductors:
When a silicon crystal is doped with a trivalent impurity e.g. aluminum, boron, indium, or gallium.
Three valence electrons of the impurity atom form three covalent bonds with the silicon atoms, there is a missing electron to form the fourth covalent bond with the fourth neighbor silicon atom.
In other words, there is a vacancy in that bond. This vacancy is called the hole and is considered to have a positive charge.
This vacancy can accommodate an electron. Such a semiconductor is called a p-type semiconductor.
The impurity atom in this case is called the acceptor impurity because it can accept an electron from a nearby silicon atom thus creating a hole in the valence band.
Difference between Intrinsic and Extrinsic Semiconductor
| Intrinsic Semiconductors | Extrinsic Semiconductors |
|---|---|
| The purest form of Semiconductor | It is the impure form of Semiconductor |
| The conductivity is very low | The conductivity is very high than an intrinsic semiconductor. |
| There is a narrow band gap between the conduction and valence bands | The energy band gap is greater than that of an intrinsic semiconductor. |
| Fermi level can be found in the forbidden energy gap. | The presence of a Fermi level depends on the type of extrinsic semiconductor. |
| Electrical conductivity determines by Temperature only. | Electrical conductivity in a pure semiconductor is affected by temperature as well as the quantity of impurity doping. |
| Pure Germanium and Silicon crystalline forms are examples. | Impurities such as arsenic, antimony, aluminum, and others are doped with Germanium and Silicon atoms. |
Frequently Asked Questions(FAQs)
Why extrinsic semiconductors are better than intrinsic ones?
Extrinsic semiconductors are better in conductivity than intrinsic semiconductors. The conductivity of extrinsic semiconductors depends on temperature as well as impurity concentration. Unlike intrinsic semiconductors, extrinsic semiconductors are of two types: p-type and n-type.
Why extrinsic semiconductors are needed?
Extrinsic semiconductors are materials that have been doped with specific impurities in order to change and improve their electrical properties. These semiconductors are then used in electronic devices such as diodes and transistors.
What are the Common Extrinsic semiconductor examples?
Examples of extrinsic semiconductors are pure silicon and germanium doped with chemical impurities like As, P, Bi, Sb, In, B, Al, etc.
What are the Common intrinsic semiconductor examples?
Intrinsic semiconductors are made of materials like silicon and germanium. They’re used most often in the manufacturing of transistors, diodes, and other electronic components.







Leave a Reply