Mendelevium-Discovery, Properties, And Applications
Mendelevium is a synthetic element with the symbol Md and atomic number 101. It is named after the Russian chemist Dmitri Mendeleev, who is known for his contributions to the periodic table.
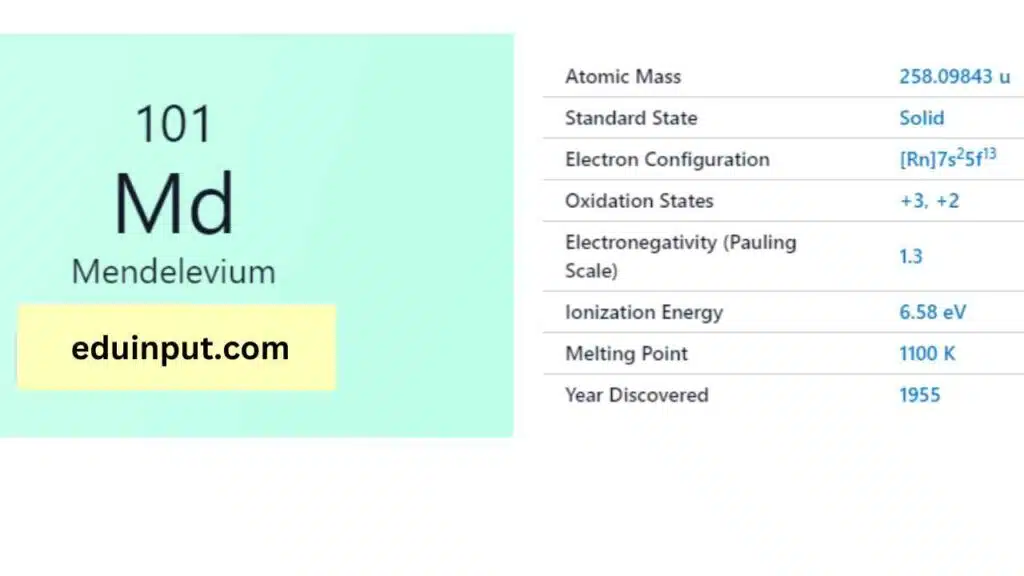
| Property | Value |
| Name | Mendelevium |
| Symbol | Md |
| Atomic number | 101 |
| Relative atomic mass (Ar) | (longest-lived isotope) |
| Standard state | Presumably a solid at 298 K |
| Appearance | Unknown, but probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Actinoid |
| Period in periodic table | 7 (actinoid) |
| Block in periodic table | f |
| Shell structure | 2.8.18.32.31.8.2 |
| CAS Registry | 7440-11-1 |
Discovery
Mendelevium was first synthesized in 1955 by a team of American scientists led by Albert Ghiorso. They bombarded a target of einsteinium-253 with alpha particles, resulting in the creation of mendelevium-256.
Physical Properties
Mendelevium is a silvery-white metal that is highly radioactive and can only be produced in small quantities. Its melting point is 827°C, and its boiling point is unknown.
Chemical Properties
Due to its high radioactivity, mendelevium has limited chemical research. However, it is believed to be a member of the actinide series and to have similar properties to other actinides. It is highly reactive with oxygen and can form oxides.
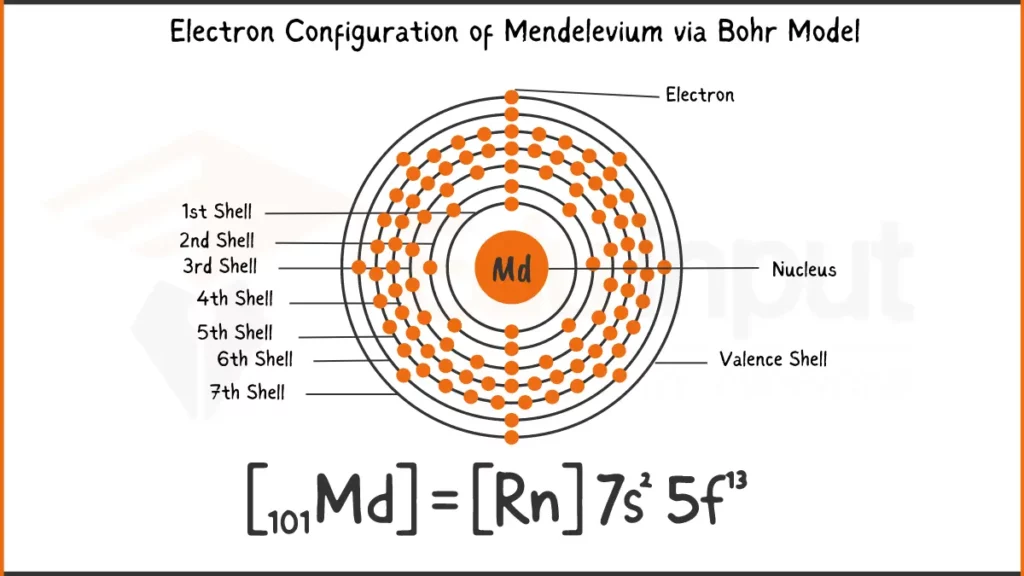
Electronic Configuration of Mendelevium
Mendelevium (Md) has 101 electrons with a configuration of [Rn] 5f¹³ 7s². This means its inner shells resemble Radon, while a specific set of electrons fills special 5f and outermost 7s subshells.
Electronic Configuration of Mendelevium via Bohr Model
Electronic Configuration of Mendelevium via Aufbau Principle
Facts
- Mendelevium is one of the rarest elements on Earth.
- Its isotopes have half-lives ranging from microseconds to several hours.
- Mendelevium has no known biological role.
Applications
Mendelevium has no practical applications and is mainly used for scientific research, such as studying the properties and behavior of heavy elements.
Mendelevium is a rare and highly radioactive element that has limited practical uses. Its discovery and subsequent research have contributed to our understanding of the properties and behavior of heavy elements.







Leave a Reply