Neon-Discovery, Properties, And Applications
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas and is the second lightest noble gas in the periodic table.

| Property | Value |
| Name | Neon |
| Symbol | Ne |
| Atomic number | 10 |
| Relative atomic mass (Ar) | Period in the periodic table |
| Standard state | Gas at 298 K |
| Appearance | Colourless |
| Classification | Non-metallic |
| Group in periodic table | 18 |
| Group name | Noble gas |
| Block in the periodic table | 2 |
| Block in periodic table | p |
| Shell structure | 2.8 |
| CAS Registry | 7440-01-9 |
Discovery
Neon was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Travers. They were studying liquefied air and found that when they evaporated the liquid, a small amount of gas was left behind. This gas was identified as neon.
Physical Properties
Neon is a colorless, odorless, and tasteless gas. It is the second lightest noble gas and is less dense than air. It has a boiling point of -246.1°C and a melting point of -248.6°C. Neon is a very stable element and does not form compounds easily.
Chemical Properties
As a noble gas, neon is inert and does not react with other elements. It has eight electrons in its outermost shell, making it stable and not likely to form chemical bonds.
Electronic Configuration of Neon
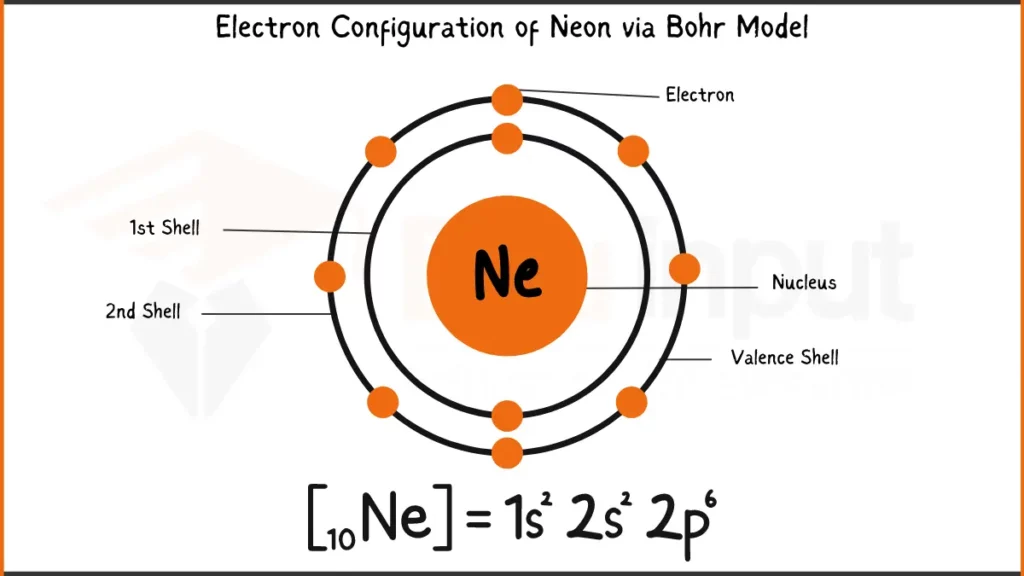
Neon (Ne), a noble gas known for its characteristic red glow in signs, has 10 electrons. Its electronic configuration is 1s²2s²2p⁶. In a simpler form, neon fills its first two shells with 2 electrons each (1s and 2s). The outermost shell (2p) holds the remaining 6 electrons.
Electronic Configuration of Neon via Bohr Model
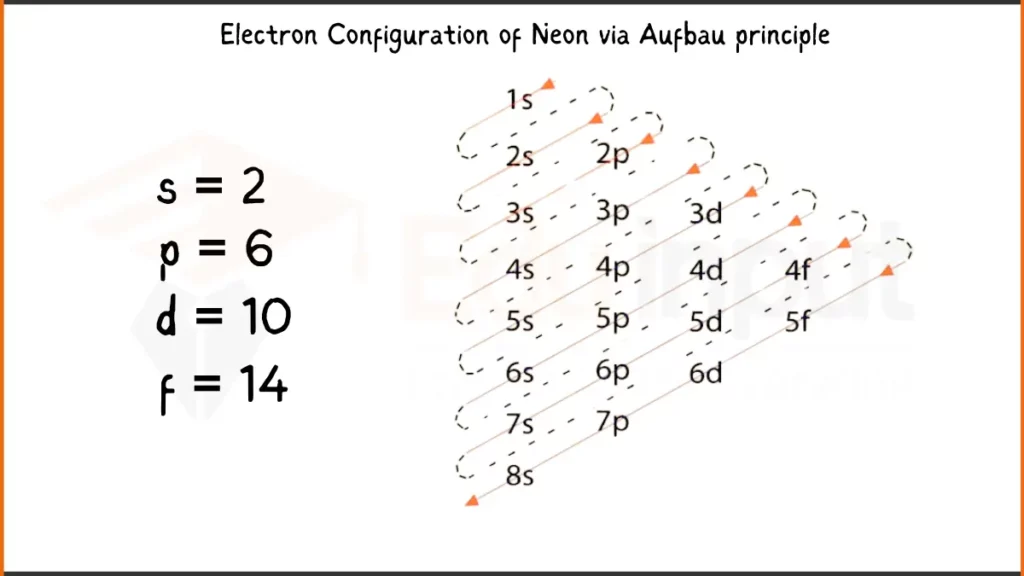
Electronic Configuration of Neon via Aufbau Principle
Facts
- Neon gets its name from the Greek word “neos,” meaning new.
- Neon is used in neon signs, where it emits a bright orange-red light.
- It is also used in gas lasers and high-voltage discharge tubes.
- Neon is the fifth most abundant element in the universe but is relatively rare on Earth.
Applications
The most well-known use of neon is in neon signs, where the gas is used to create a bright orange-red light. Neon is also used in gas lasers and in vacuum tubes. It is used as a cryogenic refrigerant and in some types of ionization detectors. In the medical field, neon is used in some types of laser surgery.






Leave a Reply