Nickel-Discovery, Properties, And Applications
Nickel is a chemical element with the symbol Ni and atomic number 28. It is a silvery-white, lustrous metal that belongs to the transition metals. Nickel is a relatively rare element on Earth and is primarily obtained from the mineral pentlandite, as well as from meteorites.
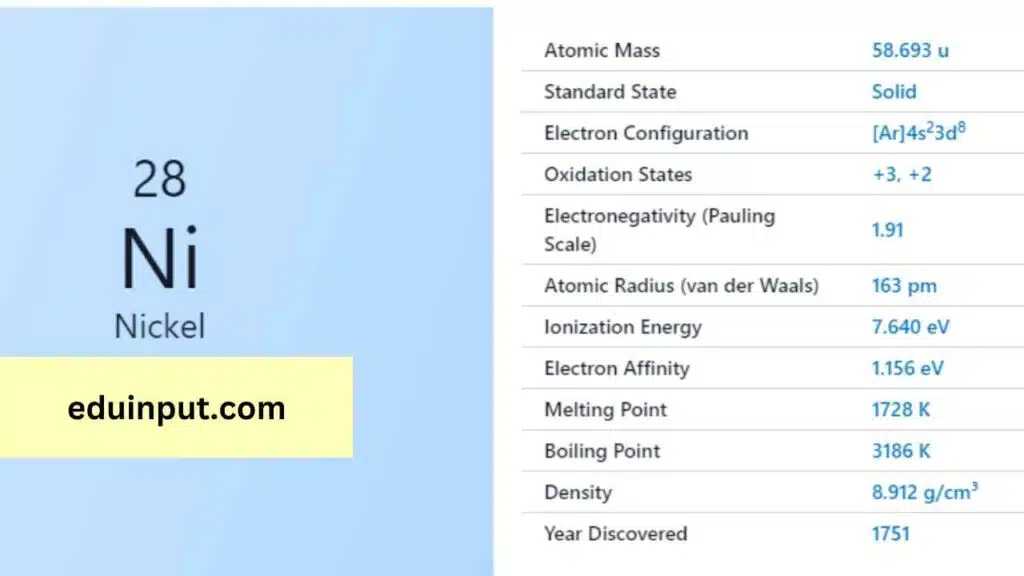
| Property | Value |
| Name | Nickel |
| Symbol | Ni |
| Atomic number | 28 |
| Relative atomic mass (Ar) | 58.6934 r |
| Standard state | Solid at 298 K |
| Appearance | Lustrous, metallic, silvery tinge |
| Classification | Metallic |
| Group in periodic table | 10 |
| Group name | (none) |
| Block in the periodic table | 4 |
| Group in the periodic table | d |
| Shell structure | 2.8.16.2 |
| CAS Registry | 7440-02-0 |
Discovery
Nickel was first discovered by Swedish chemist Axel Fredrik Cronstedt in 1751. He was able to isolate nickel from a mineral called niccolite, which had been previously mistaken for copper ore. Cronstedt named the new element after the mineral’s name, which is derived from the German word “kupfernickel” meaning “devil’s copper” or “false copper”.
Physical Properties
Nickel is a hard, ductile, and malleable metal that is resistant to corrosion and oxidation. It has a melting point of 1,453 °C (2,647 °F) and a boiling point of 2,732 °C (4,950 °F). Nickel has magnetic properties and is a good conductor of electricity and heat.
Chemical Properties
Nickel is a relatively unreactive metal and does not corrode easily in air or water. It forms a passive oxide layer on its surface that protects it from further corrosion. However, nickel can react with acids and is soluble in hydrochloric and sulfuric acids. Nickel is also capable of forming alloys with other metals, such as iron and copper.
Facts
- Nickel is a relatively rare element on Earth, accounting for only 0.008% of the Earth’s crust.
- Nickel is an essential element for many living organisms, including humans, as it is a component of several enzymes.
- Nickel is used in a wide range of applications, including coins, stainless steel production, and batteries.
- Exposure to high levels of nickel can be harmful to human health and can cause skin irritation, respiratory problems, and cancer.
Applications
Nickel has a wide range of applications in various industries. One of the most common uses of nickel is in the production of stainless steel, which accounts for approximately 65% of global nickel consumption. Nickel is also used in the production of coins, as it is a durable and corrosion-resistant metal. Additionally, nickel is used in the manufacturing of batteries, magnets, and electronics.
Nickel is a silvery-white, lustrous metal that is relatively rare on Earth. It has a wide range of applications in various industries, including stainless steel production, coinage, and electronics. Nickel is also an essential element for many living organisms and has some health risks associated with exposure to high levels.





Leave a Reply