Octet rule-Definition, Explanation, examples, exceptions
Chemistry has been one of the most interesting subjects for students. There are many concepts that students will learn while studying chemistry. But sometimes it is really difficult to remember some important concepts.
Octet rule is one of the most important concepts in the chemistry. If you are new in this subject then it is very hard to understand what is it and what is its importance. So, let’s discuss in detail what is octet rule in chemistry?.
Octet rule definition
The tendency of atoms to attain a maximum of eight electrons in the valence shell (except ‘d’ electrons) is called octet rule.
Explanation of octet rule
The octet rule is very important in chemical bonding. When two atoms form a bond, they attain an inert gas configuration with an octet of electrons i.e. ns2np6. However, in H2 molecule each of the two H- atoms attain 1s2 configuration. The completion of the octet gives rise to stability.
Important points about Octet Rule
(i) Noble gases (except He) have complete octet and are chemically inert.
(ii) The bonding in small covalent molecules is frequently achieved by sharing electrons with surrounding atoms ex. CH4, H2O.
(iii) The ions formed by electropositive and electronegative elements are generally with a compete octet e.g. Na+, Ca2+, O2-, Cl– .
(iv) Inert gas configuration is attached by losing or gaining electrons under the octet rule
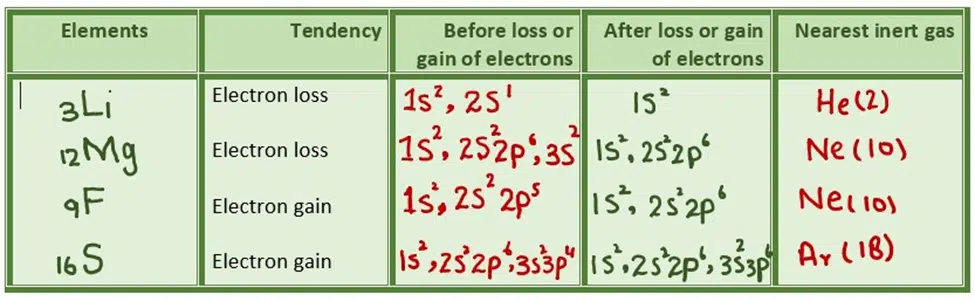
In some cases, both the tendencies have been found in NaH, hydrogen gains electrons, and in Hl. It loses electrons. It depends upon the conditions in which chemical combination takes place and the nature of elements making bonds.
Octet rule exceptions
There are many elements, which do not obey this rule. They have either incomplete octets or expansion of the octets. So this rule could not be made universal.
(a) Incomplete octets
The central atoms in the molecules like BeCl2, BF3, and NO do not have complete octets.
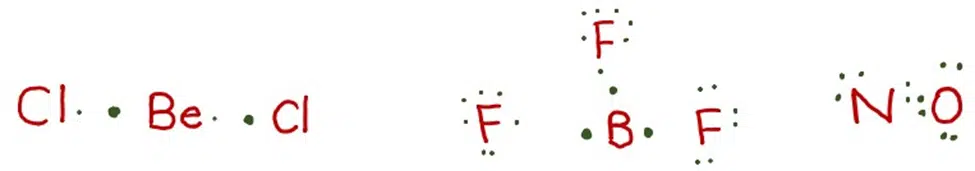
The atoms of Be, B, and N have 4, 6, and 4 electrons respectively after making bonds.
(b) Expansion of octet
In the molecules of PCL5 and SF6, the central atoms have more than eight electrons. Phosphorus and Sulphur have 10 and 12 electrons, respectively in these compounds.






Leave a Reply