Second Law of Thermodynamics- Definition, Example, And Equation
Second Law of Thermodynamics state that the entropy of a closed system always increases. The second law of thermodynamics is a law of thermodynamics that tells about heat and loss in its conversion.
The second law of thermodynamics
The first law of thermodynamics only tells that heat can be converted into an equivalent amount of work. But it does not tell the Conditions under which this conversion takes place. The second law tells us the condition Under which the heat can be converted into work and also the direction of the flow of heat.
The second law of thermodynamics equation
Mathematically, the second law of thermodynamics is expressed as:
Δ Suniv > 0
Where Δ Suniv is the change in entropy of the universe. Entropy is a measure of the randomness of a system or a measure of energy or chaos within an isolated system. It can be viewed as a quantitative indicator of energy quality.
On the other hand, there are a few factors that increase entropy in closed systems. First, in a closed system of constant mass, heat exchange with the environment takes place. This change in heat capacity introduces a perturbation into the system and increases the entropy of the system.
Second, internal changes in the molecular motions of the system can occur. This leads to disturbances that cause further irreversibility within the system, leading to an increase in its entropy.
Working on a heat engine
A heat engine absorbs heat Q1 from the hot reservoir at temperature T1. It does work W and expels heat Q2 to the low-temperature reservoir at temperature T2. As the working substance under a cyclic process, returns to its initial state, the change in internal energy is zero. Hence, from the first law of thermodynamics
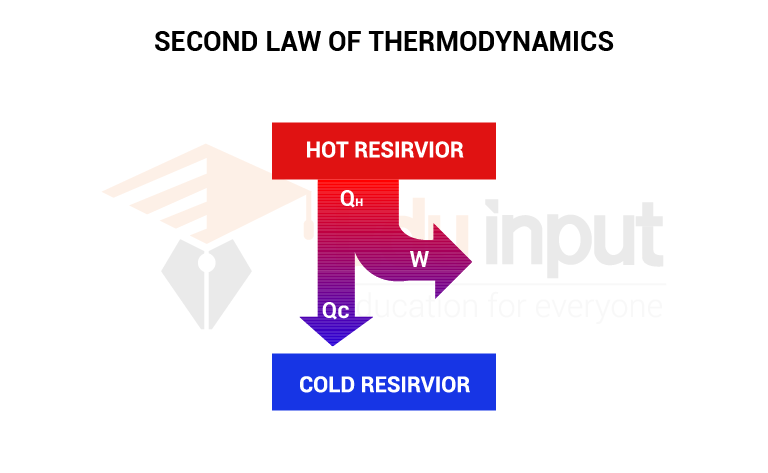
ΔQ=ΔU+W
ΔQ=W
W=Q1-Q2
The second law of thermodynamics Example:
The petrol engine takes heat from the burning fuel, converts a part of it into work, and rejects the remaining heat into the atmosphere. The efficiency of a petrol engine is 25% and that of a diesel engine is 35 to 40%. The 2nd law of thermodynamics can be stated in a number of ways. Here we shall state only Kelvin’s statement.
Kelvin’s statement
It is impossible to devise a process that may convert heat, extracted from a single reservoir, entirely into work without leaving any change in the working system.
According to Kelvin, a sink is necessary for the working of a heat engine. It means that a heat engine cannot work with a source only. Thus, two bodies at different temperatures are essential for the conversion of heat into work. This is the reason that we cannot utilize the heat contents of oceans and the atmosphere because we do not have a temperature reservoir lower than any one of the two.




Leave a Reply