Selenium-Discovery, Properties, And Applications
Selenium is a non-metal element that has the atomic number 34 and the symbol Se. It is part of the chalcogen group on the periodic table, along with oxygen, sulfur, and tellurium. Selenium is an essential nutrient for humans and animals, but it is toxic in large amounts. It is also used in various industrial and technological applications.
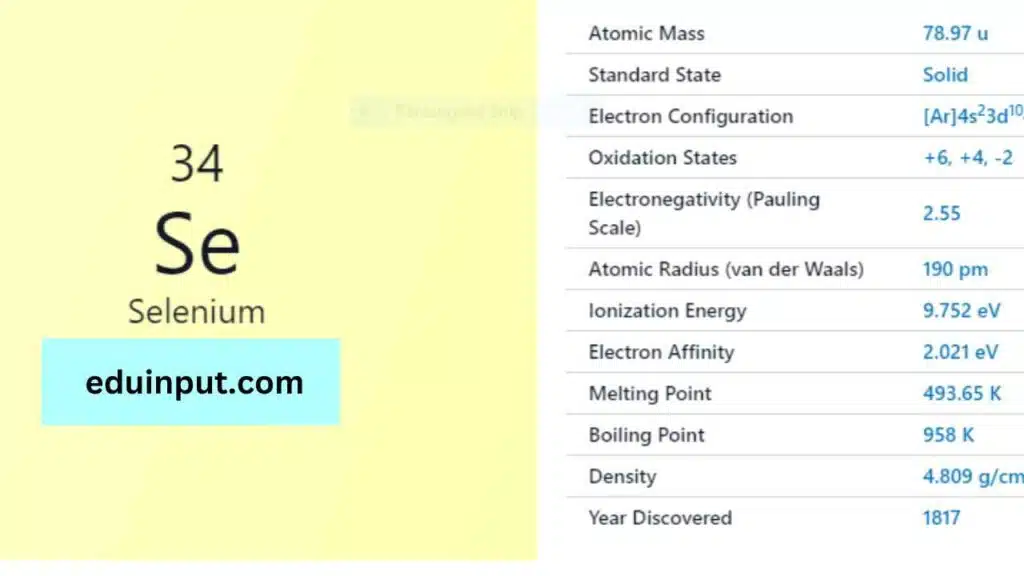
| Property | Value |
| Name | Selenium |
| Symbol | Se |
| Atomic number | 34 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Grey, metallic lustre |
| Classification | Non-metallic |
| Block in the periodic table | 16 |
| Group name | Chalcogen |
| Period in periodic table | 4 |
| Period in the periodic table | p |
| Shell structure | 2.8.18.6 |
| CAS Registry | 7782-49-2 |
Discovery
Selenium was discovered in 1817 by the Swedish chemist Jöns Jakob Berzelius. He was studying a rare mineral called kupfernickel (now known as niccolite), which was found in a mine in the Harz Mountains of Germany. Berzelius noticed that the mineral contained a new element that he named selenium, after the Greek word for moon, because of its resemblance to tellurium, which had been named after the Latin word for earth.
Physical Properties
Selenium is a non-metal element that exists in several allotropic forms. The most common form is a gray, metallic-looking solid that has a hexagonal crystal structure. It is a brittle material that can be easily crushed into a powder. Selenium has a melting point of 217°C and a boiling point of 684.9°C. It is a poor conductor of electricity and heat, but it becomes a good conductor when exposed to light.
Chemical Properties
Selenium is a highly reactive element that readily combines with other elements to form compounds. It reacts with oxygen to form selenium dioxide (SeO2) and selenium trioxide (SeO3), which are used in the production of glass and ceramics. Selenium also forms compounds with hydrogen, sulfur, and halogens. Selenium compounds are used in the production of pigments, rubber, and other materials.
Facts
- Selenium is an essential nutrient for humans and animals. It is found in foods such as Brazil nuts, tuna, and beef.
- Selenium is used in the production of photovoltaic cells, which convert sunlight into electricity.
- Selenium is also used in the production of glass and ceramics, as well as in the metallurgical industry.
- Selenium has been shown to have antioxidant properties, which may help to protect against cancer and other diseases.
Applications
Selenium has a wide range of applications in industry, technology, and medicine. Some of its most common uses include:
- Photovoltaic cells: Selenium is used in the production of photovoltaic cells, which convert sunlight into electricity.
- Glass and ceramics: Selenium compounds are used in the production of glass and ceramics to improve their clarity and strength.
- Metallurgy: Selenium is used in the production of stainless steel, as well as in the refining of copper, lead, and other metals.
- Agriculture: Selenium is an essential nutrient for plants, and it is used as a fertilizer in agriculture.
- Medicine: Selenium supplements are used to treat and prevent certain diseases, including cancer and heart disease.




Leave a Reply