Building Blocks of Matter: Atoms, Subatomic Particles, & Quarks
Elementary particles are the building blocks of matter. Subatomic particles, including protons, neutrons, and electrons, make up atoms. Atoms are the basic building blocks of all matter. These atomic building blocks combine to form elements, which create everything in the universe.
Atoms are the building blocks of matter and consist of subatomic particles. The building blocks of protons and neutrons are quarks. A quark atom is smaller than an atom. The 4 fundamental forces govern the interactions of these particles.
Building Blocks of Matter
If you look at glass, the visible matter is made up of different materials, and the invisible material of the glass is actually called atoms. Atoms are the basic building blocks of matter and are composed of subatomic particles, which include protons, neutrons, and electrons. Atoms are the building blocks of all matter and are the foundation of every physical substance.
Protons have positive charges, neutrons have no charge, and electrons have negative charges. These three subatomic particles form the atomic nucleus. When these three subatomic parts of an atom are joined together, they create a central core, which is the atom. The particles that surround the central core are called electrons. Atoms with subatomic particles are essential in defining the structure of matter.
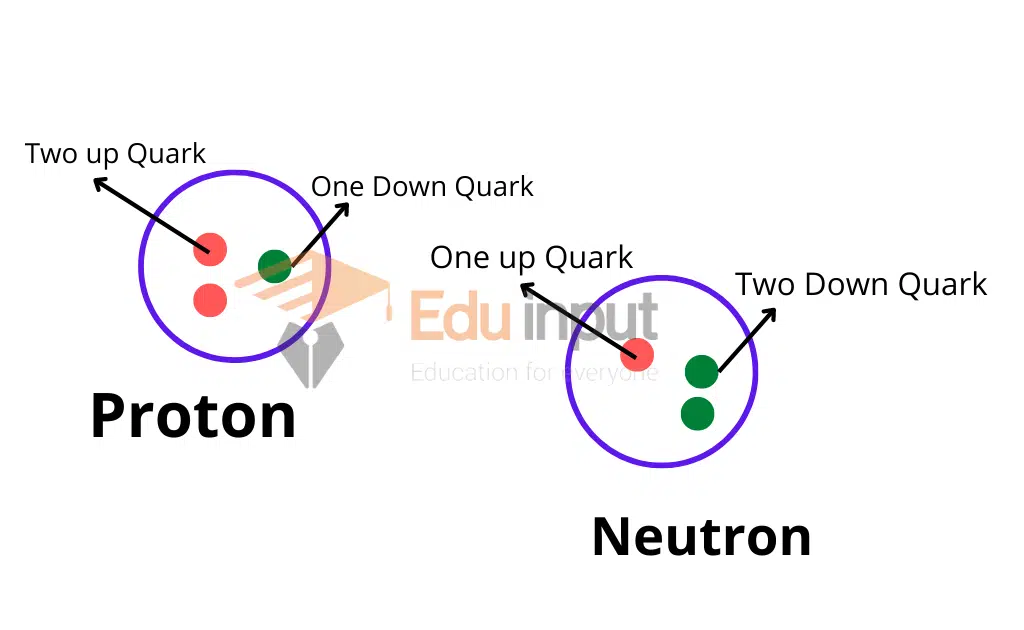
Protons, neutrons, and electrons are the basic building blocks of matter. These atomic building blocks are further made up of quarks. A proton consists of two up quarks and one down quark, whereas a neutron consists of one up quark and two down quarks. Quarks are subatomic particles smaller than atoms and are the fundamental building blocks of protons and neutrons.
Other substances like oxygen, nitrogen, carbon, hydrogen, and sulfur are made up of these basic building blocks. The atomic structure of helium, for example, consists of two protons, two neutrons, and two electrons. The diagram of subatomic particles shows how atoms, the building blocks of matter, function.
Sub-Atomic Particles
Subatomic particles are the basic building blocks of matter and can be divided into three main groups:
- Photons
- Leptons
- Hadrons
Elementary particles are the fundamental constituents of all matter. Photons and leptons are considered elementary particles, while hadrons are composed of even smaller subatomic particles called quarks. Scientists now categorize all matter into two fundamental groups: quarks and leptons.
Hadrons
Hadrons are particles that experience the strong nuclear force, one of the four fundamental forces in nature. They include:
- Protons and neutrons, which are the building blocks of atoms
- Mesons, which are subatomic particles lighter than protons
Particles with a mass equal to or greater than protons are classified as baryons, while those lighter than protons are called mesons.
Leptons
Unlike hadrons, leptons do not interact via the strong nuclear force. Important leptons include:
- Neutrinos, nearly massless particles that interact weakly
- Electrons, which are crucial for atomic structure
- Muons, heavier relatives of electrons
Quarks
In chemistry, quarks are the fundamental subatomic particles that form protons and neutrons, which are the building blocks of atoms. Quarks in chemistry define the fundamental structure of atoms and matter. Since quarks are smaller than atoms, they play a key role in determining the properties of matter at a submicroscopic level.
What Are Quarks?
A quark is a subatomic particle that serves as the building block of protons and neutrons. The meaning of quark in chemistry is a fundamental subatomic particle that carries a fractional electric charge and combines to form protons and neutrons, which make up atomic nuclei. Scientists classify quarks into six types:
- Up quark
- Down quark
- Strange quark
- Charm quark
- Bottom quark
- Top quark
A proton consists of two up quarks and one down quark, while a neutron is made up of one up quark and two down quarks. This explains why quarks are the subatomic particles that form atomic nuclei.
Quark vs. Atom: What’s the Difference?
When comparing quarks vs. atoms, it’s important to understand that:
- Quarks are smaller than atoms and exist inside protons and neutrons.
- Atoms are made up of subatomic particles, including protons, neutrons, and electrons.
- Subatomic particles like quarks do not exist independently in nature; instead, they combine to form larger atomic building blocks.
Sub-Atoms and Sub-Particles of an Atom
Since quarks are subatomic particles, they are sometimes referred to as sub-atoms or sub-particles of an atom. Without quarks, protons and neutrons could not exist, making them essential to the structure of matter.
Currently, the hundred of hadrons can be accounted for in terms of six quark and their anti-quarks. It is believed that quarks cannot exist on their own t existence has been indirectly verified.






Leave a Reply