Atomic Nucleus- Nucleons, Charge Number, and Mass Number
The atomic nucleus is the dense region that consists of protons and neutrons at the center of an atom. The atomic nucleus was discovered by Ernest Rutherford in 1911.
Atomic Nucleus
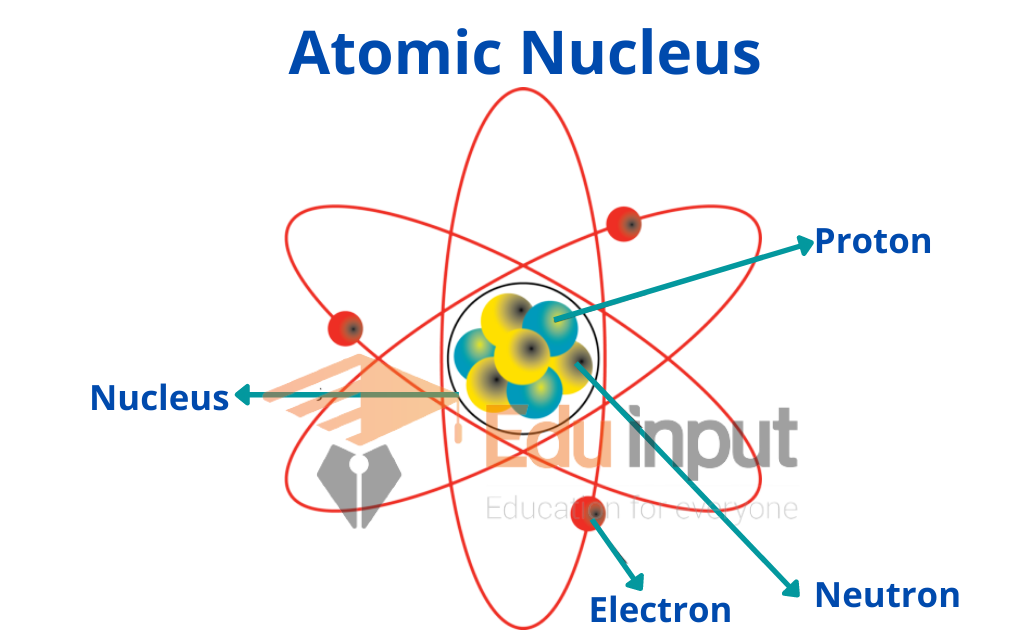
At the center of each and every atom, there is an infinitesimally small nucleus. The entire positive charge of the atom and about 99.9 percent of its mass is concentrated in the nucleus.
The tininess of the nucleus can be imagined by comparing that the radius of the atom is 10 times the radius of the nucleus.
Nucleons
A nucleus consists of nucleons comprising protons and neutrons. A proton has a positive charge equal to 1.6×10-19 kg and its mass is 1.673 x 10-27 kg.
A neutron has no charge on it, but its mass is 1.675x 10-27kg.
The mass of a neutron is almost equal to the mass of the proton.
To indicate the mass of atomic particles, instead of the kilogram, a unified mass scale (u) is generally used.
By definition, 1u is exactly one-twelfth the mass of a carbon atom (1 u=1.6606 x 10-27 kg).
In this unit
Mass of a proton = 1.007276 u
Mass of a neutron =1.008665 u
Mass of an electron is =0.00055 u
The charge on a proton is equal in magnitude to the charge on an electron. The charge on the proton is positive while that of an electron is negative.
As an atom on the whole is electrically neutral, therefore, we can conclude that the number of protons inside the nucleus is equal to the number of electrons outside the nucleus.
Charge Number
The number of protons inside a nucleus is called the atomic number or the charge number of an atom.
It is denoted by Z. Thus the total charge of any nucleus is Ze here ‘e’ indicates the charge on one proton.
Mass Number
The combined number of all the protons and neutrons in a nucleus is known as its mass number and is denoted by A.
The number of neutrons N present in a nucleus is given by
N= (A-Z)
It can be understood by considering different elements of the periodic table.
Atomic Nucleus Example
The hydrogen atom is the simplest of all the atoms. Its nucleus is composed of only one proton, that is for hydrogen A = 1, Z=1.
That is why hydrogen is represented by the symbol 11H. Next in the periodic table after the hydrogen element is the helium element. Its nucleus contains two protons and two neutrons. This means for helium A = 4 and Z = 2 and hence helium is represented as 42He.
Uranium – is a heavy element of the periodic table. The charge number Z of uranium is 92 while its mass number A is 235. This is represented as 23592U.
It has 92 protons while the number of neutrons N is given by the equation N = A-Z=235-92=143.
In this way, the number of protons and neutrons in atoms of all the elements of the periodic table can be determined.
It has been observed that the number of neutrons and protons in the initial light elements of the periodic table is almost equal but in the later heavy elements, the number of neutrons is greater than the number of protons in the nucleus.




Leave a Reply