Thermal Energy-Definition, Types, And Examples
Thermal energy is the energy contained within a system that is responsible for the temperature. The flow of heat is referred to as thermal energy. Thermodynamics is a branch of physics that deals with how heat is transferred between different systems and how work is done in the process
What is thermal energy?
The movement of particles within the body or system causes the body or system to have thermal energy. It is one of the different types of energy, which refers to the ability to do work. As such thermal energy can be defined as the ability of something to work as a result of the movement of its particles.
Thermal energy is the energy that an object or body possesses by virtue of the movement of its particles. The total internal energy of an object is the result of the random motion of its atoms and molecules. The movement of particles results in thermal energy which is a type of energy. The energy possessed by an object due to its motion is called kinetic energy.
Thermal energy from friction
Thermal energy produces due to friction. There is an example of a man pushing a box across a rough floor. The thermal energy of the box-floor system is not stored as potential energy since the work done is not conservative. The box and floor are raised in temperature by the thermal energy flowing from the box to the floor.
Thermal energy from drag
An example of a non-conservative force is the drag on a moving object caused by air or water. There would still be some residual motion of the fluid if the object were to stop moving. This wouldn’t last long after some time. The large-scale motions of the fluid are eventually re- distributed into many smaller random motions of the molecule in the fluid. The increased thermal energy in the system is represented by the motions.
Types of Thermal Energy
Depending on how internal energy is transferred from one body to another, thermal energy can be classified into various types. The following are the different types.
Depending on how internal energy is transferred from one body to another, thermal energy can be classified into Three types.
1. Conduction
This type of thermal energy involves the movement of the particles of an object, without the movement of the body itself. It can be seen in all phases of liquid, gas, and solid. The movement of the particles of an object causes an increase in thermal energy which is transferred by contact with the neighboring atoms and Molecules within the object.
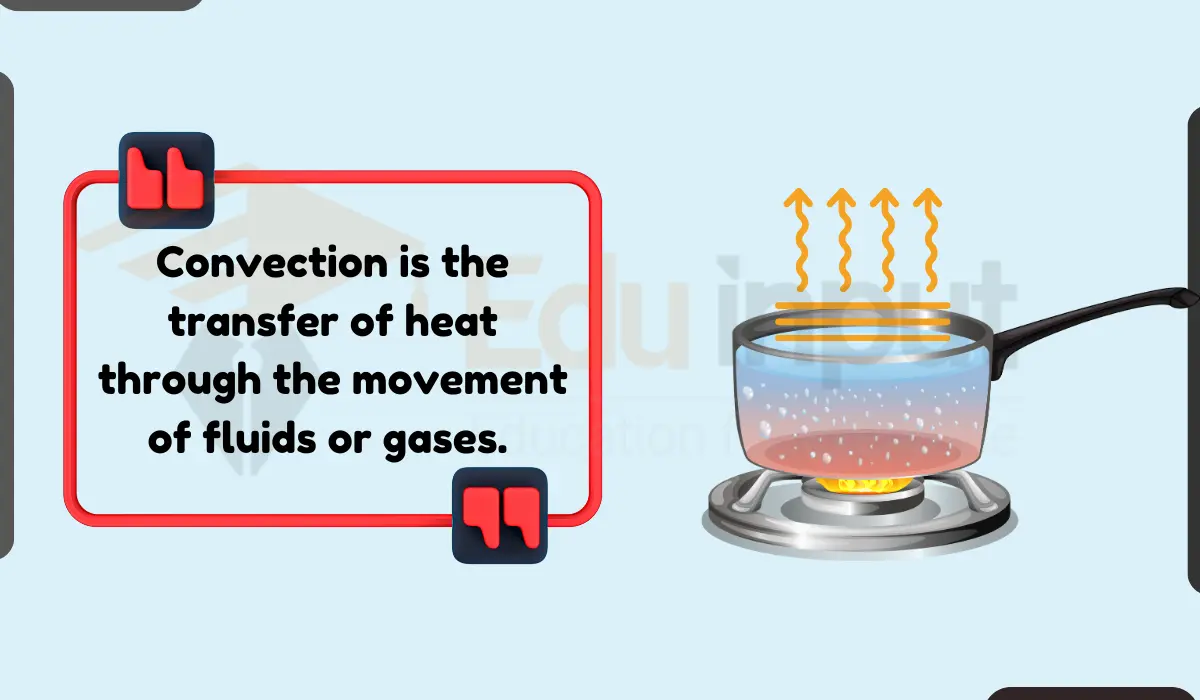
2. Convection
Between two bodies brought into contact, convection occurs, not only within a body but also between them. The fluid motion will most definitely occur if one of the substances is a liquid or gas. convection is the transfer of energy between a solid surface and a moving liquid or gas.
3. Radiation
The substances exchanging heat don’t need to be in contact with each other in this type of thermal energy, which is fundamentally different from the other types. Despite being separated by a vacuum, thermal energy can be transferred between two bodies by radiation.
Examples of Thermal Energy
There are different examples of thermal energy
- Solar Energy
- Geothermal Energy
- Heat Energy From the Oceans
- Fuel Cell Energy
- A Cup of hot Milk
- Melting Ice






Leave a Reply