Valence Bond Theory, introduction, postulates, examples and limitations
Introduction
The theory according to the valence electrons present in the outermost orbital overlap with the orbitals of the bonding atoms. In this way, the electronic cloud of the shared electron pair has a maximum density between the two nuclei.
Postulate of VBT
(i) A covalent bond is formed due to the overlap of the partially filled atomic orbitals.
(ii) In overlapping orbitals, electrons become paired with opposite spin to stabilize them.
(iii) Larger the overlap, more is the energy released, and the stronger will be the bond.
(iv) Essential condition to form a bond is that atomic orbitals must overlap.
(v) The overlapping orbitals must have the same symmetry to the bond axis.
(vi) The direction of the bond is determined by the direction of the overlapping orbitals.
Examples
All those elements which form covalent bonds with other elements have valence electrons in different types of orbitals. They can also do the overlapping of orbitals.
(i) Molecule of hydrogen H2 (s-s overlapping)
The simplest example is the formation of the hydrogen molecule. Symmetrical and spherical 1s orbital of one H atom overlaps with other 1s orbital. The electron density is concentrated in the region of their meeting point (i.e. between the two nuclei).
(ii) Molecule of HF.
S-orbital of the H-atom overlaps with the 2Pz orbital of the F-atom.
(iii) Molecule of F2.
Each F-atom has one electron in a 2Pz orbital. They do the overlapping to produce a sigma bond between F atoms.
Basic definitions
Unhybridized atomic orbitals
The atomic orbitals which have individual shapes and energy are called an unhybridized atomic orbital. e.g., s, p, d, f.
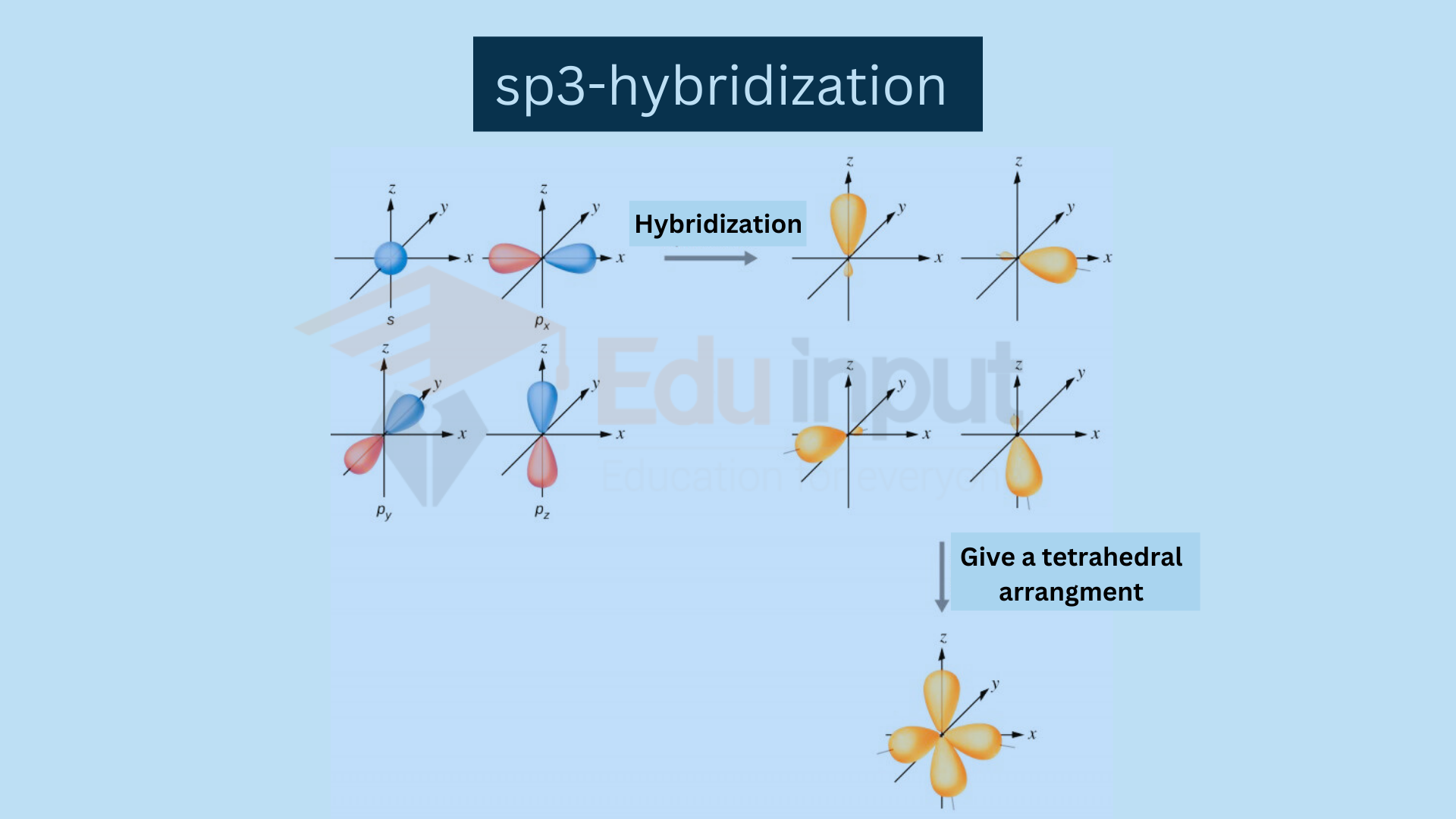
Hybrid Atomic Orbitals
The orbitals produced by the hybridization having the same shape and energy but different orientations in space are called hybrid atomic orbitals e.g., sp3, sp², sp, etc.
Central Atom
The multivalent atom present in the center of the molecule bonded to two or more atoms is called a central atom. In CH4 and NH3 C and N are central atoms.
Bond Angle
The angle between the two bonds is known as the bond angle.
Bond Pair
The electron pair which is shared between two bonded atoms is called a bond pair H2, BeCl2, BCI3, and CH4 have one, two, three, and four bond pairs.
Molecular Geometry
The pattern or arrangement of atoms around the central atom is called molecular geometry.
Electronic Geometry
The arrangement of electron pairs around the central atom is called electron geometry. It is the geometry of the central atom.
Localized Electrons
The shared pair of electrons which is localized between the nuclei of two bonded atoms is called localized electrons. In CH2 = CH2 electrons are localized between carbon atoms.
Delocalized Electrons
The shared electrons that extend over more than two nuclei of bonded atoms are called delocalized electrons. In benzene, π -electrons are delocalized over six carbon atoms.
Sigma (σ) Bond
The bond which is formed by the head-to-head or linear overlap of atomic orbitals is called a sigma bond.
π-Bond
The bond which is formed by the parallel or sideways overlap of atomic orbitals is called π -bond. e.g., in CH2= CH2 there is one σ and one π-bond.
Limitations of Valence Bond Theory
Inadequate Treatment of Excited States
VBT struggles to adequately describe the electronic structure of molecules in excited states. It does not seamlessly accommodate cases where electrons are promoted to higher energy levels.
Limited Treatment of Molecular Spectra
VBT has limitations in predicting and explaining molecular spectra, particularly electronic spectra associated with transitions between different energy levels.
Neglect of Molecular Orbital Theory
VBT does not fully incorporate the concepts of Molecular Orbital Theory, which provides a more comprehensive view of electron distribution and bonding.
Challenges in Transition Metal Complexes
VBT faces challenges in explaining the bonding in transition metal complexes due to the involvement of d-orbitals, which are not explicitly considered in traditional VBT.

 written by
written by 



Leave a Reply