What is a molecular ion? introduction, types and examples
A molecular ion is formed when electrons are added to or removed from a molecule. When electrons are removed, the ion gains a net positive charge, while the addition of electrons will give a net negative charge to the molecular ion.
For example, CH4+ , N2+ , SO42– etc.
How molecular ions are formed?
When the vaporized organic sample passes into the ionization chamber of a mass spectrometer, it is bombarded by a stream of electrons. These electrons have a high enough energy to knock an electron off an organic molecule to form a positive ion.
Types of molecular ions
There are two types of molecular ions cationic and anionic molecular ions.
What are cationic molecular ions?
Those molecules which carry positive charge are called cationic molecular ions. CH4+ , N2+
What are anionic molecular ions?
Those molecules which carry negative charge are called anionic molecular ions. SO42-
Why cationic molecular ions are present more abundantly than anionic molecular ion?
As we know that cationic molecular ions are formed, by the loss of electrons, and anionic molecular ions are formed by the gain of electrons. It is easier to remove an electron from a molecule than to introduce an electron into a molecule. Therefore cationic molecular ions are present more abundantly than anionic molecular ions.
Why NH4+ is not a molecular ion?
The ammonium ion is not a molecular ion because it is formed by the coordinate covalent bond between ammonia (NH3) and hydrogen ion (H+).
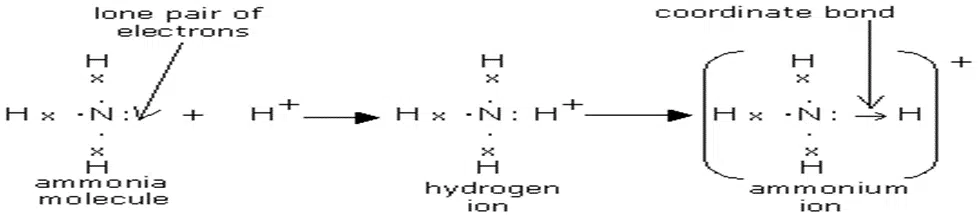
What is difference between molecule and molecular ion?
A molecule is made up of atoms of one or more elements. Some examples of molecules are oxygen gas (O2), Hydrogen gas (H2), sulfuric acid (H2SO4), carbon dioxide (CO2), and water (H2O), among others. In a molecule, the atoms are combined in a certain fixed ratio. For example, in water (H2O).
A molecular ion is formed when electrons are added to or removed from a molecule. When electrons are removed, the ion gains a net positive charge, while the addition of electrons will give a net negative charge to the molecular ion. It is called a molecular ion. CH4+ .
| Molecule | Molecular ion |
| It is the smallest particle of a substance that can exist independently. | It is formed by the loss or gain of electrons by a molecule. |
| It is a stable unit. | It is reactive specie. |
| It is formed by the combination of atoms. | It is formed by the ionization of molecules. |
| It is always neutral. | It can have a negative or positive charge |




Leave a Reply