What is a molecule in chemistry? Eduinput
Molecules are the smallest units of substance that can exist independently.
These are very important for the survival of any living organism. The human body contains millions of molecules and each molecule is a very small particle.
The molecule is the smallest particle of a pure substance that can exist independently. It may consist of two or more atoms.
Here are the molecules examples…
For exampleH2,H2O, CO2, C6H12O6 etc.
Why molecules can exist independently?
Molecules can exist independently because molecules are formed by the combination of atoms. Actually, atoms react together to attain noble gases’ electronic configuration.
Noble gases are stable because their outermost shells are complete. In another word, noble gases obey the duplet and octet rule. So when a molecule is formed it becomes stable and exists independently.
What are molecules made of?
Molecules are formed when the electrons of the element are separated and they become stable. They are made up of two or more atoms bonded together. The atoms bond together through the electrons present in each atom.
The number of electrons in a particular atom is termed valence. The valence determines the types of bonds that are formed between atoms. The different kinds of bonds in molecules are covalent, ionic, and metallic. The number of bonds that are present depends upon the nature of the atoms present.
What is duplet rule?
Attaining of two electrons in the outermost shell of an atom is called the duplet rule.
For example, Helium obeys the duplet rule. The atomic number of helium is two. It contains two electrons in its outermost shell.

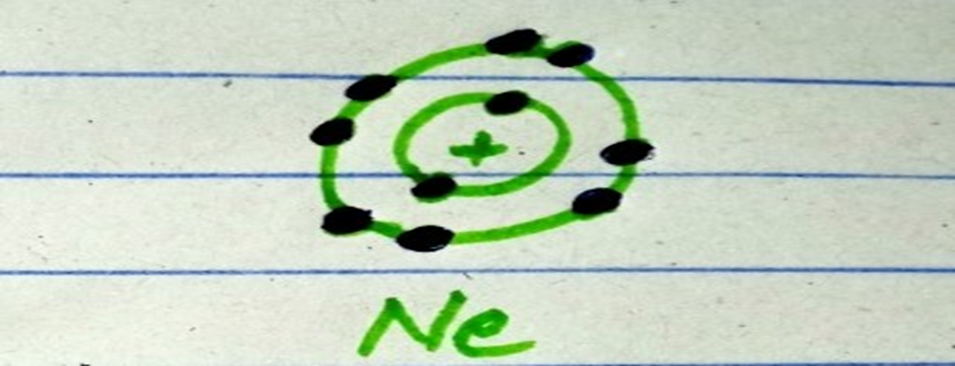
What is octet rule?
Attaining eight electrons in the outermost shell of an atom is called the octet rule.
For example, Neon obeys the octet rule. The atomic number of neon is ten. It means that neon contains two electrons in the first shell and eight electrons in the outermost shell.
What is atomicity?
The number of atoms present in a molecule is called its atomicity.
The atomicity of carbon dioxide (CO2) is three because carbon dioxide contains three atoms in its molecule. Similarly the atomicity of NH3, CH4, and C6H12O6is respectively 4,5 and 24.
Classification of molecules
Molecules are classified into three categories on the basis of atomicity, nature, and size. Let us explain them one by one.
- On the bases of atomicity (number of atoms)
- Mono atomic molecules
Those molecules which contain only one atom are called mono-atomic molecules.
For example He, Ne, Ar, Kr,Xe etc.
Further, the ones based on the number of atoms per molecule are Diatomic, Triatomic, and Tetratomic molecules.
Poly atomic molecules
Those molecules which contain many atoms are called polyatomic molecules.
For example C6H12O6, C12H22O11etc.
- On the bases of the Nature of atoms
- Homo atomic molecules
Those molecules which consist of atoms of the same element are called homo atomic molecules.
For example H2, Cl2, O3, P4, S8 etc.
Hetero atomic molecules
Those molecules which consist of atoms of different elements are called heteroatomic molecules.
For example H2O, CO2, C6H12O6 etc.
On the bases of the Sizes of molecules
Micromolecules
Those molecules which are small in size are called micromolecules.
For example O3, H2O, CO2, C6H12O6 etc.
Macromolecules
Those molecules which are large in size are called macromolecules.
For example Hemoglobin, Diamond Graphite, etc.
What is hemoglobin molecule?
- It is a blood protein.
- It helps to carry oxygen from our lungs to all parts of our body.
- The elements present in hemoglobin are C,H,O,N along with Fe.
- Each molecule of hemoglobin is made up of nearly 10,000 atoms.
- Each hemoglobin molecule is 68,000 times heavier than a hydrogen atom.
Molecules are also categorized on the basis of dissolving properties. For example polar and non-polar molecules.
What do polar and non-polar mean?
In simple terms, polar means oppositely charged, and non-polar means equally charged. To understand the difference between polar and non-polar bonds, it is essential to comprehend electronegativity.
What are polar molecules?
Molecules that are formed when atoms with different electronegativities share electrons to form a covalent bond. Polar bonds have a high melting point, boiling point, and low vapor pressure. Polar molecules interact with other polar substances.
Polar molecules are soluble in polar solvents like water. These molecules have positive and negative charges on opposite ends. Therefore, they are electrically charged. Examples: hydrochloric acid.
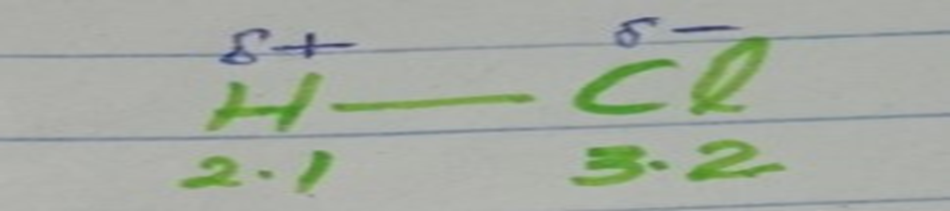
What are non polar molecules?
Non-polar molecules are formed when two atoms share electrons evenly, a type of chemical bond known as a non-polar covalent bond is created.
Non-polar molecules are soluble in non-polar solvents like carbon tetrachloride. Non-polar bonds have low melting points, boiling points, and high vapor pressure. Examples: Hydrogen.

What is a molecule?
A molecule is a group of two or more atoms that are chemically bonded together, representing the smallest fundamental unit of a chemical compound.
What is the difference between a molecule and an atom?
An atom is the basic unit of a chemical element, while a molecule is made up of two or more atoms chemically bonded together.
How are molecules formed?
Molecules are formed when two or more atoms come together and form chemical bonds. These bonds can be ionic or covalent depending on the nature of the atoms involved.
What is the importance of understanding molecules in chemistry?
Understanding molecules is crucial in chemistry because all chemical reactions involve the breaking and forming of chemical bonds between molecules. Knowing the properties and behavior of different molecules helps in predicting and understanding the outcome of chemical reactions.
What are the different types of molecules?
Molecules can be classified into different types based on the nature of the chemical bonds that hold them together. These include covalent molecules, ionic molecules, and metallic molecules.
What is a covalent molecule?
A covalent molecule is a molecule that is formed by the sharing of electrons between two or more atoms. These molecules can be either polar or nonpolar depending on the electronegativity of the atoms involved.
What is an ionic molecule?
An ionic molecule is a molecule that is formed by the transfer of electrons between two or more atoms. These molecules are usually made up of a positively charged ion and a negatively charged ion.
What is a metallic molecule?
A metallic molecule is a molecule that is made up of metal atoms that are bonded together in a lattice structure. These molecules are characterized by their high electrical conductivity and malleability.
How can we predict the shape of a molecule?
The shape of a molecule can be predicted using VSEPR theory, which stands for Valence Shell Electron Pair Repulsion theory. This theory states that the shape of a molecule is determined by the repulsion between the electron pairs in the valence shell of the central atom.




Leave a Reply