Yttrium-Discovery, Properties, And Applications
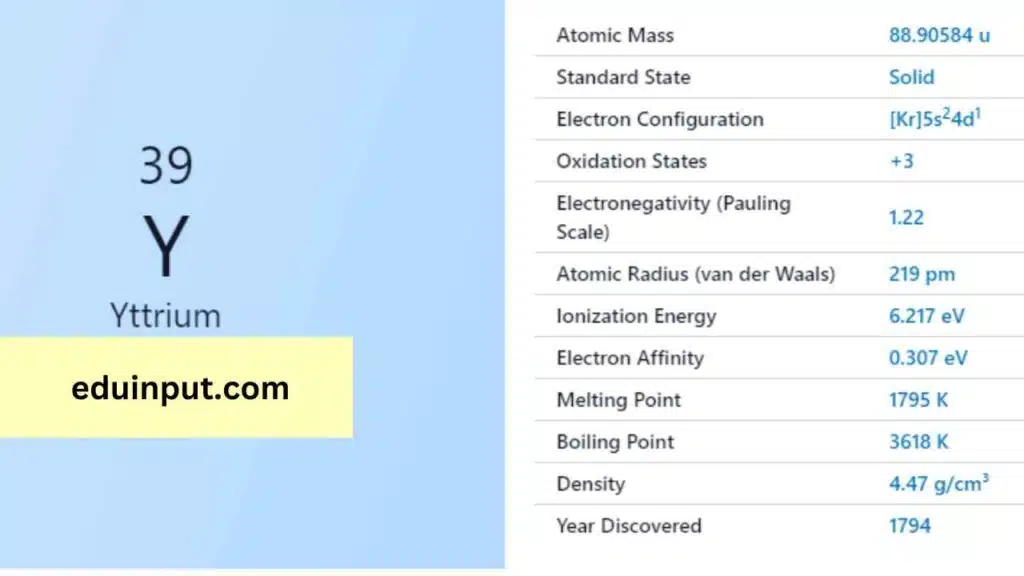
Yttrium is a chemical element with the symbol ‘Y’ and atomic number 39. It is a rare earth metal that has a unique set of properties that make it useful in a variety of applications, from electronics to medical imaging. Despite its rarity, yttrium has become an important element in modern technology.
Discovery
Yttrium was first discovered in 1794 by Swedish chemist Johan Gadolin, who found it in a mineral called ytterbite. The mineral was named after the village of Ytterby, Sweden, where it was found. Yttrium is found in various minerals and ores and is mainly extracted from the mineral monazite.
Physical Properties
Yttrium is a silvery-white metal that is relatively soft and ductile. It has a density of 4.47 g/cm3, a melting point of 1,522°C, and a boiling point of 3,338°C. Yttrium is also a paramagnetic element, which means that it is attracted to magnetic fields.
Chemical Properties
Yttrium is a relatively reactive element that readily forms compounds with other elements. It reacts with oxygen in the air to form yttrium oxide, and with acids to form yttrium salts. Yttrium also has a high affinity for oxygen and is often used as a scavenger in metallurgical processes to remove impurities from metals.
Facts
- Yttrium is a rare earth element that is found in small quantities in various minerals and ores.
- Yttrium is often alloyed with other metals, such as aluminum and magnesium, to improve their mechanical properties.
- Yttrium is a key element in high-performance materials used in electronics, medical imaging, and other applications.
Applications
Yttrium has a wide range of applications in various fields, including electronics, medical imaging, and nuclear energy. Some of the major applications of yttrium include:
- Electronics: Yttrium is used in the production of high-performance electronic components, such as superconductors and microwave filters. Yttrium oxide is also used as a dielectric material in capacitors and other electronic devices.
- Medical imaging: Yttrium is used in the production of medical isotopes, such as yttrium-90, which is used in radiation therapy to treat cancer. Yttrium-90 is also used in medical imaging to help diagnose and treat various diseases.
- Nuclear energy: Yttrium is used in the production of fuel rods for nuclear reactors. It is also used as a neutron absorber to control nuclear reactions.
Yttrium is a rare earth element that has become increasingly important in modern technology. Its unique properties, such as its ability to improve the mechanical properties of other metals and its use in medical imaging, make it an essential element in a wide range of applications. As technology continues to advance, the demand for yttrium is likely to increase, making it an important element for the future.
| Property | Value |
| Name | Yttrium |
| Symbol | Y |
| Atomic number | 39 |
| Relative atomic mass (Ar) | 88.90584 (2) |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Group in periodic table | 3 |
| Group name | (none) |
| Period in periodic table | 5 |
| Block in periodic table | d |
| Shell structure | 2.8.18.9.2 |
| CAS Registry | 7440-65-5 |





Leave a Reply