Experimental Verification of dual Nature of Matters
Who verified the Dual nature of matter?
The dual nature of matter got its verification in 1927 by Davison and Germer. They are American scientists. They produced the electrons from heated tungsten filament and accelerated the electrons by applying potential differences.
They proved that their accelerated electrons diffraction like waves when they fall on the nickel crystal. So, they verified the wave nature of electrons.
BOHR’S THEORY AND WAVY NATURE OF ELECTRONS
According to the fourth postulate of Bohr’s model, the angular momentum of a moving electron is an integral multiple of h/2π.
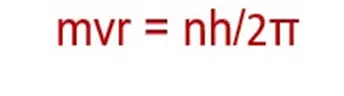
This equation had no theoretical background in 1913 when Bohr gave his atomic model. Let us prove this equation from de Broglie’s equation.
If the electron has waves and has to move around the nucleus in a circular orbit, then the circumference of the circle is an integral multiple of the wavelength as shown in the following diagram:
The concept of integral multiples of wavelength is deduced from the concept of standing waves. Stretched strings have a standing wave. The electron revolving around the nucleus also has standing waves.
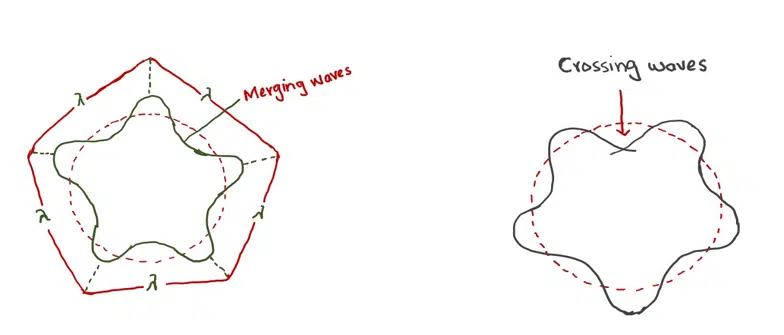
de Broglie’s electron of wave extending round the nucleus and accommodate in Bohr’s circular orbit
(a) Electron wave in phase having n= 5 (n= No. of wavelength). Constructive interference of electron waves.
(b) Electron wave out of phase- Destructive- interference of electron waves.
If r is the radius of the orbit and λ is the wavelength associated with the electron, then the circumference of the circle is an integral multiple of the wavelength.
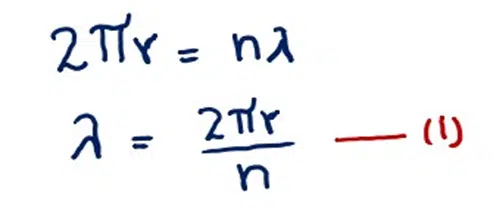
We also know that
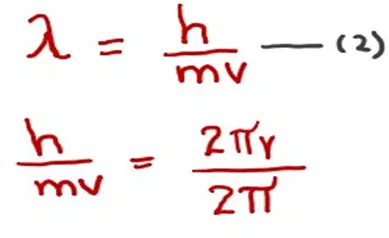
Hence,
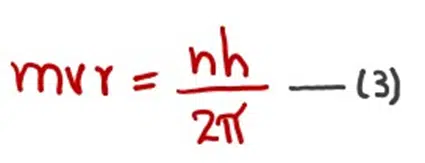
When ‘mvr’ is not the whole number multiple of h/2π, the electron wave is said to be out of phase. In other words, we can say that if the circumference of Bohr’s orbit, 2πr is bigger or smaller than ‘nλ’ then the electron wave will not extend around the nucleus in a circular orbit.



Leave a Reply