What is Lattice Point in chemistry and How to find it
As a chemistry student, you may have come across the term “lattice point” in your studies of crystal structures. In this article, we will explore what a lattice point is and how it relates to the formation of crystals.
What is lattice point in a crystal?
A lattice point is a point in space where atoms, ions, or molecules are positioned in a repeating pattern to form a crystal lattice. These points represent the locations where the particles in the crystal structure are positioned, and the pattern of these points determines the overall structure of the crystal.
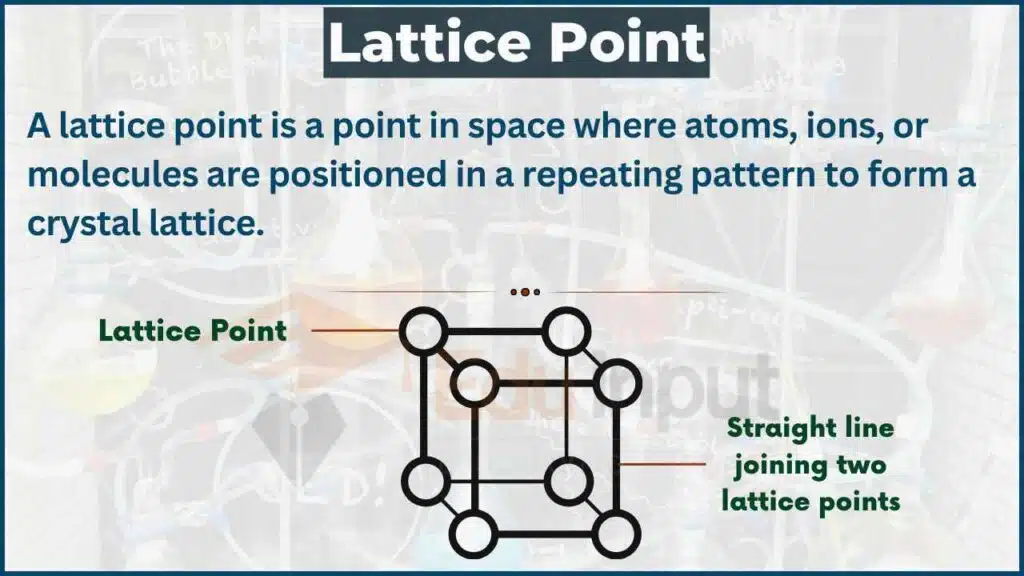
In a crystal lattice, the particles are arranged in a regular pattern, and each particle is located at a lattice point. The lattice points themselves are not particles, but rather they represent the positions where the particles are located.
The arrangement of lattice points in a crystal can be described using the concept of unit cells. A unit cell is the smallest repeating unit of a crystal lattice, and it contains one or more lattice points. By repeating the unit cell in all three dimensions, we can generate the entire crystal structure.
The lattice points in a crystal are held together by various intermolecular forces, such as ionic bonds, covalent bonds, and van der Waals forces. These forces determine the overall strength and stability of the crystal lattice.
One important property of a crystal lattice is its symmetry. The arrangement of lattice points in a crystal can exhibit various types of symmetry, such as rotational symmetry or reflection symmetry. The symmetry of a crystal lattice can have important implications for its physical and chemical properties.
Other related terms that you may come across in the study of crystal structures include crystallography, which is the study of crystal structure and properties, and Bravais lattice, which is a mathematical concept that describes the possible arrangements of lattice points in a crystal.
How to find lattice points?
To find lattice points, you first need to understand the concept of a unit cell. A unit cell is the smallest repeating unit of a crystal lattice and contains one or more lattice points. By repeating the unit cell in all three dimensions, we can generate the entire crystal structure.
To find the lattice points within a unit cell, we can use the following steps:
- Determine the dimensions of the unit cell: The unit cell can be described using three lengths, a, b, and c, which represent the dimensions along the three crystallographic axes.
- Identify the lattice type: There are seven different types of lattices that can be formed, including simple cubic, body-centered cubic, and face-centered cubic. The lattice type will determine the positions of the lattice points within the unit cell.
- Determine the positions of the lattice points: The lattice points within a unit cell can be located using their fractional coordinates, which are given as fractions of the unit cell dimensions. For example, a lattice point at the corner of a unit cell in a simple cubic lattice will have fractional coordinates of (0, 0, 0).
- Repeat the unit cell: Once the positions of the lattice points within a unit cell have been identified, the entire crystal lattice can be generated by repeating the unit cell in all three dimensions
In conclusion, lattice points are an important concept in the study of crystal structures. They represent the locations where particles are positioned in a repeating pattern to form a crystal lattice. By understanding the arrangement of lattice points, we can better understand the physical and chemical properties of crystals.





Leave a Reply