Covalent solids- types, formation, properties, crystal structure and uses
What are Covalent Solids?
Covalent solids are a type of solid material that is held together by covalent bonds.
Covalent solid definition
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In covalent solids, the atoms are tightly bonded to each other, creating a strong and rigid structure. The nature of these bonds gives covalent solids unique properties such as high melting points and hardness.
Examples of covalent solids include diamond, graphite, and silicon carbide. These materials exhibit different atomic arrangements and bonding forces, resulting in variations in their physical and chemical properties.
The study of covalent solids provides valuable insights into the behavior of materials and has numerous applications in fields such as electronics, optoelectronics, and materials science.
Arrangement and bonding of atoms in covalent solids
The arrangement of atoms in covalent solids is highly organized and repetitive, forming a regular three-dimensional pattern known as a crystal lattice. This lattice structure is responsible for the solid’s stability and distinct properties.
The bonding between atoms in covalent solids results from the overlapping of orbitals, leading to the formation of strong localized covalent bonds. This bonding mechanism allows the solid to maintain shape and resist deformation under external forces.
Depending on the nature of the covalent bonds, covalent solids can be classified into different categories, such as molecular solids, network solids, and metallic solids. Each category exhibits unique atomic arrangements that contribute to their specific properties and behavior.
Understanding the atomic structure and bonding arrangement in covalent solids is crucial for elucidating their properties and exploring their various applications in industry and technology.
Types of Covalent Solids
There are two types of covalent solids, network covalent solids and molecular covalent solids.
1. Network Covalent Solids
- Definition: Each atom is covalently bonded to neighboring atoms, forming a continuous three-dimensional network.
- Characteristics:
- Strong and stable structures.
- High melting points.
- Generally, it’s very hard.
Example: Diamond
Each carbon atom is bonded to four other carbon atoms in a tetrahedral arrangement.
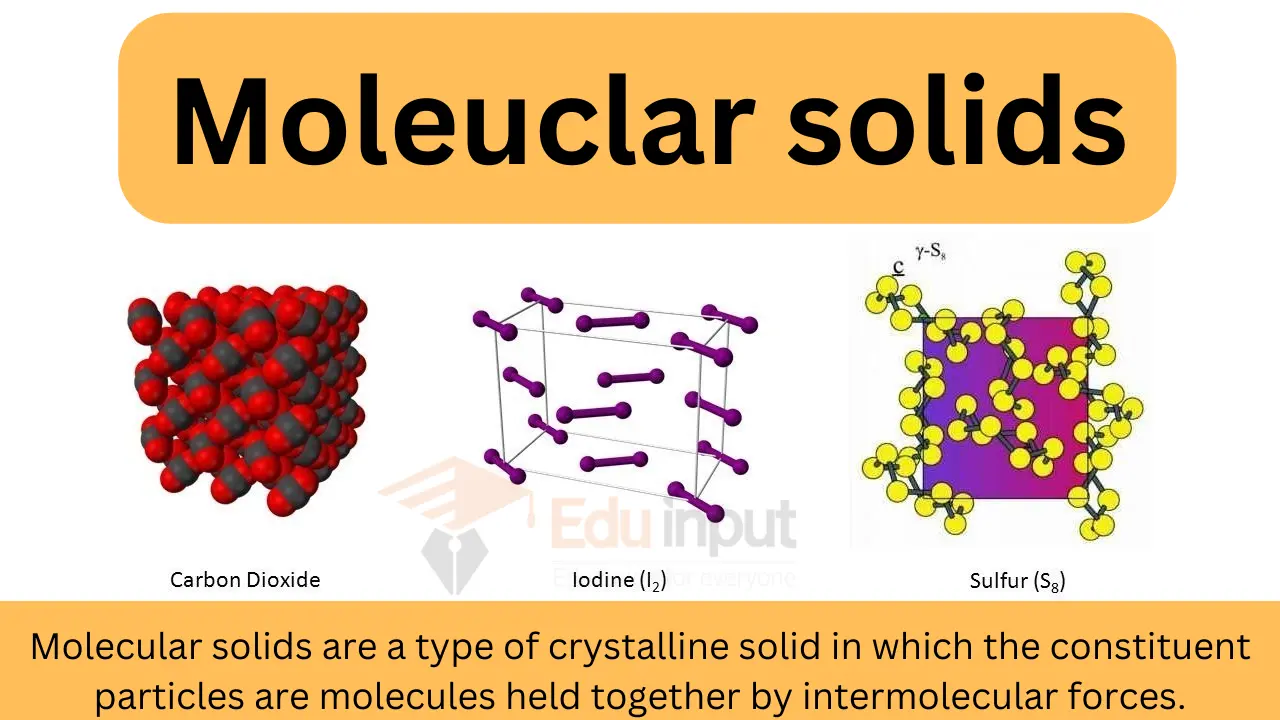
2. Molecular Covalent Solids
- Definition: Discrete molecules are held together by intermolecular forces such as van der Waals forces or hydrogen bonding.
- Characteristics:
- Individual molecules may be polar or nonpolar.
- Lower melting points compared to network covalent solids.
- Usually softer.
- Example: Solid Iodine
- Weak intermolecular forces hold individual iodine molecules together.
Properties of Covalent Solids
Covalent solids possess diverse physical and chemical properties
1. High Melting and Boiling Points:
Due to strong covalent bonds within the solid, it requires a significant amount of energy to break these bonds. It Results in high stability, allowing covalent solids to remain solid at elevated temperatures.
2. Insolubility in Water and Polar Solvents:
- Covalent solids are generally insoluble in water and other polar solvents.
- Covalent bonds involve electron sharing, resulting in a balanced charge distribution.
- Less susceptible to interactions with polar molecules.
- Soluble in nonpolar solvents such as hexane or benzene.
3. Nature of Covalent Bonds
- Covalent bonds are formed by the sharing of electrons between atoms.
- This sharing leads to a balanced charge distribution in covalent solids.
- Contributes to their resistance to dissolution in polar solvents.
4. Versatile Solubility
- Covalent solids exhibit solubility in nonpolar solvents.
- This property is utilized in various applications, from organic chemical synthesis to the production of advanced materials.
5. Stability at Elevated Temperatures
- The high melting and boiling points contribute to the stability of covalent solids.
- Allows them to maintain a solid state even when subjected to higher temperatures.
6. Applications in Advanced Materials
- Covalent solids find applications in the production of advanced materials.
- Their unique properties are valuable in industries such as organic chemistry and materials science.
Crystal Lattices in covalent solids
The lattice structures of covalent solids can be diverse, depending on the types and arrangements of atoms. One common type is the diamond structure. In diamonds, each carbon atom bonds with four neighboring carbon atoms in a tetrahedral arrangement. This makes diamonds super hard and transparent.
Another example is the quartz crystal structure, made of silicon and oxygen atoms in a repeating pattern. Quartz crystals have piezoelectric properties and are used in electronic devices. The various lattice structures in covalent solids contribute to their wide range of properties, making them crucial in many industries.
Bonding Forces in covalent solids
Covalent solids stick together because of important forces called intermolecular forces. These forces happen when the positively charged nuclei of nearby atoms interact with the negatively charged electrons they share. In covalent solids, strong bonds form a lattice structure, making them tough and resistant to heat.
A crucial intermolecular force in covalent solids is the dispersion force, also called the London dispersion force. This force comes from temporary changes in how electrons are spread out, creating temporary dipoles.
These temporary dipoles make nearby atoms or molecules develop their own temporary dipoles, creating a weak attraction between them. The strength of this force depends on the size and shape of the atoms or molecules involved. Bigger ones usually have stronger dispersion forces because they have more electrons and a larger electron cloud to influence nearby species. The dispersion force is universal in all covalent solids, no matter what they’re made of.
Thermal Conductivity
Covalent solids have special heat-related properties that set them apart from other solids. Thermal conductivity, which is the ability to conduct heat, varies in covalent solids based on their specific structure.
The strength of covalent bonds, formed by electron sharing between atoms, is a big factor influencing thermal conductivity. These bonds create a rigid structure that limits the movement of atoms and heat transfer, resulting in generally low thermal conductivity for covalent solids.
Beyond low thermal conductivity, covalent solids also show other interesting thermal traits. They tend to have high melting and boiling points because breaking the strong covalent bonds requires a lot of energy.
This stability at high temperatures distinguishes covalent solids from other types. Additionally, some covalent solids, like diamond, have unique thermal expansion behaviors. Diamond, for example, expands very little when heated, making it highly resistant to thermal stress. This property contributes to the diamond’s exceptional hardness and durability.
Applications of covalent solids
| Application | Covalent Solid | Description |
| 1. Electronics | Silicon (Si) | Covalent solids like silicon are essential in the semiconductor industry for manufacturing electronic components such as transistors and integrated circuits. |
| 2. Cutting Tools | Diamond (C) | Diamond, a covalent solid, is the hardest known material and is widely used in cutting tools for machining, drilling, and grinding due to its exceptional hardness and durability. |
| 3. Insulating Materials | Silicon Dioxide (SiO2) | Covalent solids like SiO2 (quartz) are used as insulating materials in electronics and construction due to their stable structure and resistance to electrical conduction. |
| 4. High-Temperature Ceramics | Silicon Carbide (SiC) | Silicon carbide, a covalent solid, is used in the production of high-temperature ceramics and refractory materials due to its excellent thermal and mechanical properties. |
| 5. Structural Materials | Graphite (C) | Covalent solids like graphite are used as structural materials, lubricants, and in the production of composites due to their unique layered structure and good electrical conductivity. |
| 6. Dental Materials | Hydroxyapatite (Ca5(PO4)3OH) | Covalent solids like hydroxyapatite are used in dental applications, such as in the production of dental implants and bone grafts, due to their biocompatibility and strength. |
| 7. Optoelectronics | Gallium Nitride (GaN) | Covalent solids like GaN are crucial in optoelectronics for making LEDs (light-emitting diodes) and laser diodes, benefiting from their wide bandgap and high electron mobility. |
| 8. Adhesives | Polyethylene (C2H4)n | Covalent solids like polyethylene are used in the production of adhesives and various types of plastic due to their flexible and durable nature, making them suitable for bonding applications. |
| 9. Lubricants | Teflon (Polytetrafluoroethylene, PTFE) | Covalent solids like Teflon are used as lubricants and non-stick coatings due to their low friction properties and chemical inertness. |
| 10. Biomedical Applications | Polymethyl Methacrylate (PMMA) | Covalent solids like PMMA are used in biomedical applications, such as in the production of acrylic bone cement and contact lenses, due to their biocompatibility and transparency. |






Leave a Reply