Why Is The Term Half-Life Used To Measure Radioactivity?
The term half-life is used to measure radioactivity because it represents the time it takes for the radioactivity of a substance to decrease by half due to radioactive decay. This is a convenient and accurate way to measure the decay of radioactive materials, as it provides a consistent and predictable measure of their radioactivity.
What is Half-Life?
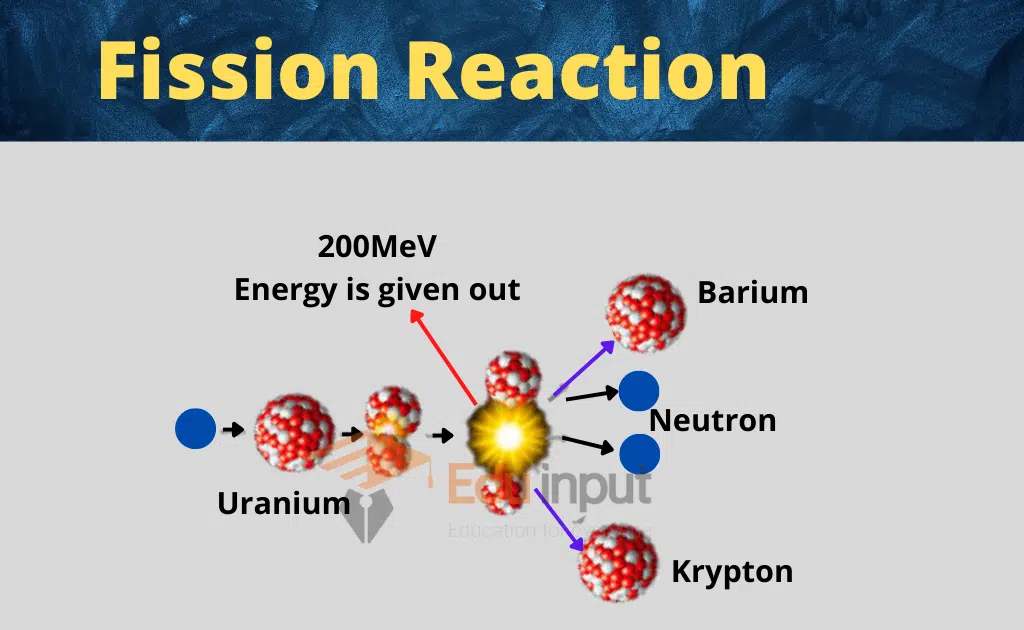
The half-life of a radioactive isotope is the time it takes for half of the atoms in a sample of that isotope to decay. This means that after one half-life, the sample will have half as much radioactivity as it did initially. After two half-lives, the sample will have one-quarter as much radioactivity, and so on.

The half-life of a radioactive isotope is a constant, and it does not depend on the initial amount of the isotope or the conditions in which it is stored. For example, a sample of cobalt-60 with a half-life of 5.27 years will always have half as much radioactivity after 5.27 years, no matter how much of the isotope is present or what temperature it is stored at.
Properties of Half-Life
- The half-life of a radioactive isotope is a constant, unchanging property of that isotope. It does not vary depending on how the isotope is stored or handled.
- The half-life of a radioactive isotope does not alter its chemical properties.
- The half-life of a radioactive isotope is not affected by its temperature or pressure.
- The half-life of a radioactive isotope is a statistical property, which means that not all of the atoms in a sample will decay at the same instant.
Why is Half-Life Used to Measure Radioactivity?
The half-life is a useful way to measure radioactivity because it is a simple and straightforward way to quantify the rate of decay. It is also independent of the initial amount of the isotope, which makes it a more reliable measure than other methods, such as counting the number of radioactive decays per second.
The half-life is also used to determine the age of objects that contain radioactive isotopes. For example, carbon-14 dating is a technique that uses the half-life of carbon-14 to determine the age of organic materials. Carbon-14 is a radioactive carbon isotope with a half-life of 5,730 years. By measuring the amount of carbon-14 in an organic material, scientists can estimate how long ago the material was alive.
Uses of Half-Life
The half-life of radioactive isotopes is also used in a variety of other applications, such as:
- Calculating the safe handling and disposal of radioactive materials
- Determining the effectiveness of radiation therapy
- Evaluating the risk of exposure to radiation
- Dating geological formations
- Studying the evolution of life




Leave a Reply