Alpha Decay-Definition, Examples, And Equation
Alpha decay is defined as the spontaneous disintegration of an atomic nucleus through the emission of an alpha particle. It is a type of radioactive decay that occurs in heavy elements with high atomic numbers. The decay process is governed by the principles of quantum mechanics and occurs due to the instability of the nucleus.
What is Alpha Decay?
Alpha decay is a process by which an unstable atomic nucleus spontaneously transforms into a more stable configuration by emitting an alpha particle. The symbol used to represent alpha decay is the Greek letter “α.” It is commonly written as a superscript to the left of the atomic symbol of the parent nucleus.
The formula for alpha decay can be expressed as:
Parent Atomic Symbol-Mass ⟶ Daughter Atomic Symbol-Mass + α
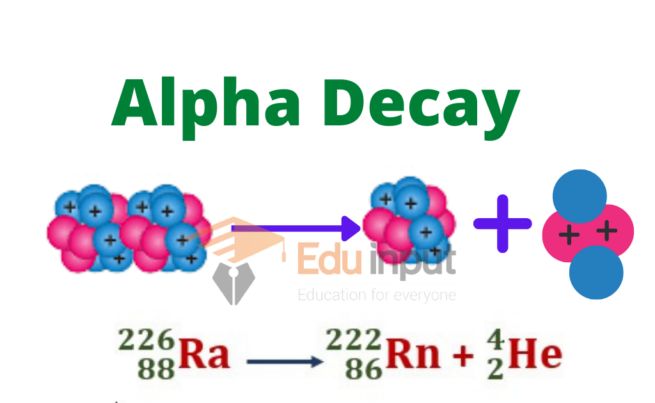
An alpha particle consists of two protons and two neutrons, making it identical to a helium-4 nucleus. This emission occurs when the nucleus has excess protons and neutrons, which leads to instability. The emission of an alpha particle reduces the atomic number of the nucleus by 2 and the mass number by 4.
To understand alpha decay, it is crucial to grasp the concept of nuclear stability. Nuclei with an excess of protons and neutrons compared to the optimal ratio tend to be unstable.
In such cases, the nucleus tries to achieve a more stable configuration by releasing excess particles, such as alpha particles. This process continues until the nucleus reaches a more balanced and stable state.
Alpha Decay Equation
The alpha decay process can be represented by the following equation:
Parent Nucleus ⟶ Daughter Nucleus + Alpha Particle
For example, a common alpha decay is the decay of uranium-238:
238U ⟶ 234Th + 4He
Here, uranium-238 decays into thorium-234 by emitting an alpha particle.
Significance of Alpha Decay
Alpha decay plays a significant role in the natural decay chains of heavy elements. It contributes to the formation of various isotopes and the radioactive series of elements, such as uranium and thorium series.
Additionally, alpha decay is essential for understanding the stability and behavior of atomic nuclei, nuclear reactions, and the creation of new elements.
Applications of Alpha Decay
Alpha decay has several practical applications. It is utilized in smoke detectors, where the decay of a small amount of radioactive material produces ionizing radiation, which triggers a current and sounds an alarm.
Alpha decay is also employed in nuclear power plants, where it is crucial for controlling and understanding nuclear reactions. Moreover, alpha decay plays a vital role in scientific research and the study of fundamental particles and their interactions.
Factors Affecting Alpha Decay
Several factors can influence the rate and occurrence of alpha decay. These factors include
- The composition of the nucleus
- The binding energy of the alpha particle
- The size of the nucleus
- The quantum mechanical tunneling effect
The interplay of these factors determines the probability and characteristics of alpha decay.
Recent Advances in Alpha Decay Research
In recent years, researchers have made significant advancements in the understanding and utilization of alpha decay. These include studies on the properties of exotic nuclei, investigation of alpha decay chains in superheavy elements, and the development of new techniques for detecting and measuring alpha particles. These advances contribute to expanding our knowledge of nuclear physics and its applications.
Related FAQ’s
What is meant by alpha decay?
Alpha decay refers to the spontaneous emission of an alpha particle from the nucleus of an atom. It is a type of radioactive decay that leads to the transformation of an unstable nucleus into a more stable configuration.
What is an alpha decay example?
An example of alpha decay is the decay of uranium-238, where it transforms into thorium-234 by emitting an alpha particle.
Where does alpha decay occur?
Alpha decay occurs in heavy elements with high atomic numbers. It is a natural process observed in various radioactive isotopes.

 written by
written by 




Leave a Reply