Endothermic Reactions-Characteristics, Identification, and Examples
Definition of Endothermic Reactions
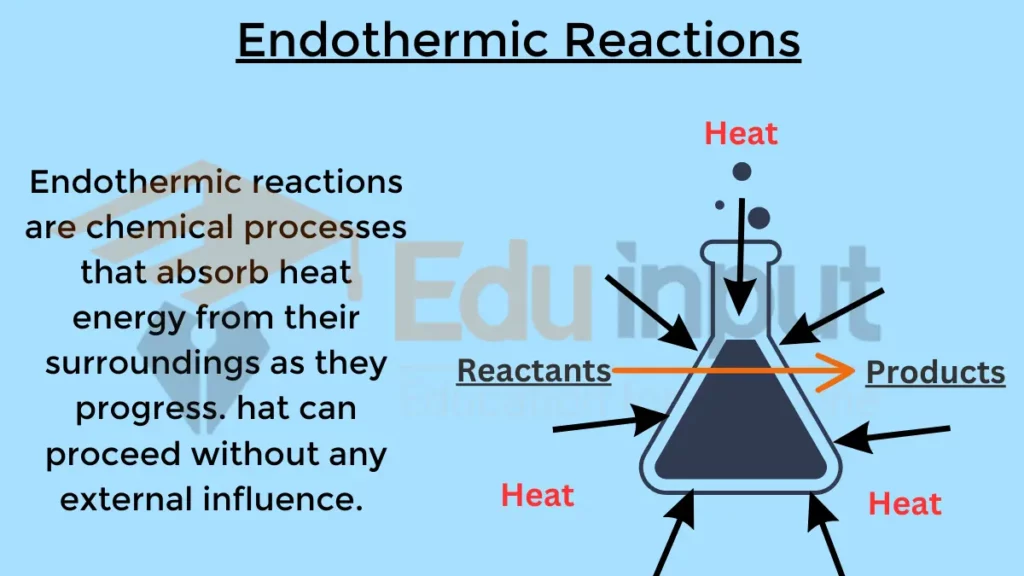
Endothermic reactions are chemical processes that absorb heat energy from their surroundings as they progress. Endothermic reactions can be spontaneous, but only if the temperature is high enough.
Unlike exothermic reactions, which release heat, endothermic reactions draw in heat from their environment, causing a decrease in temperature in the surrounding system.
Also Read: What are Non-Sponteneous Reactions
Aalso read: Difference Between Endothermic And Exothermic Reactions
Characteristics of Endothermic Reactions
Several characteristics distinguish endothermic reactions:
1. Heat Absorption
The most defining characteristic is the absorption of heat energy, resulting in a decrease in temperature in the reaction mixture or surroundings.
2. Positive ΔH
Endothermic reactions have a positive change in enthalpy (ΔH), indicating that the overall energy of the system increases as heat is absorbed.
3. Non-Spontaneity
Endothermic reactions are typically non-spontaneous under standard conditions. They require an input of energy to proceed.
Heat Changes in Endothermic Reaction
Endothermic reactions absorb heat from their surroundings, resulting in a decrease in temperature. This is because the energy absorbed is used to break the bonds in the reactants, forming new bonds in the products. Endothermic reactions have a positive change in enthalpy (ΔH), indicating that the system gains energy overall.
Endothermic reactions require an external source of energy to initiate, as they are not spontaneous under standard conditions. This is why ice packs and chemical cold packs work – they absorb heat from your skin, causing a cooling sensation.
How Endothermic Reactions Occur?
Endothermic reactions occur due to the breaking and forming of chemical bonds. In these reactions, the energy required to break the bonds in the reactants is greater than the energy released when new bonds form in the products.
The difference in energy is absorbed from the surroundings in the form of heat.
Why Endothermic Reactions Absorb Heat?
Endothermic reactions absorb heat because they require an input of energy to overcome the activation energy barrier and initiate the chemical transformation.
The energy needed to break the existing bonds in the reactants is greater than the energy released when new bonds are formed in the products, resulting in a net absorption of heat from the surroundings.
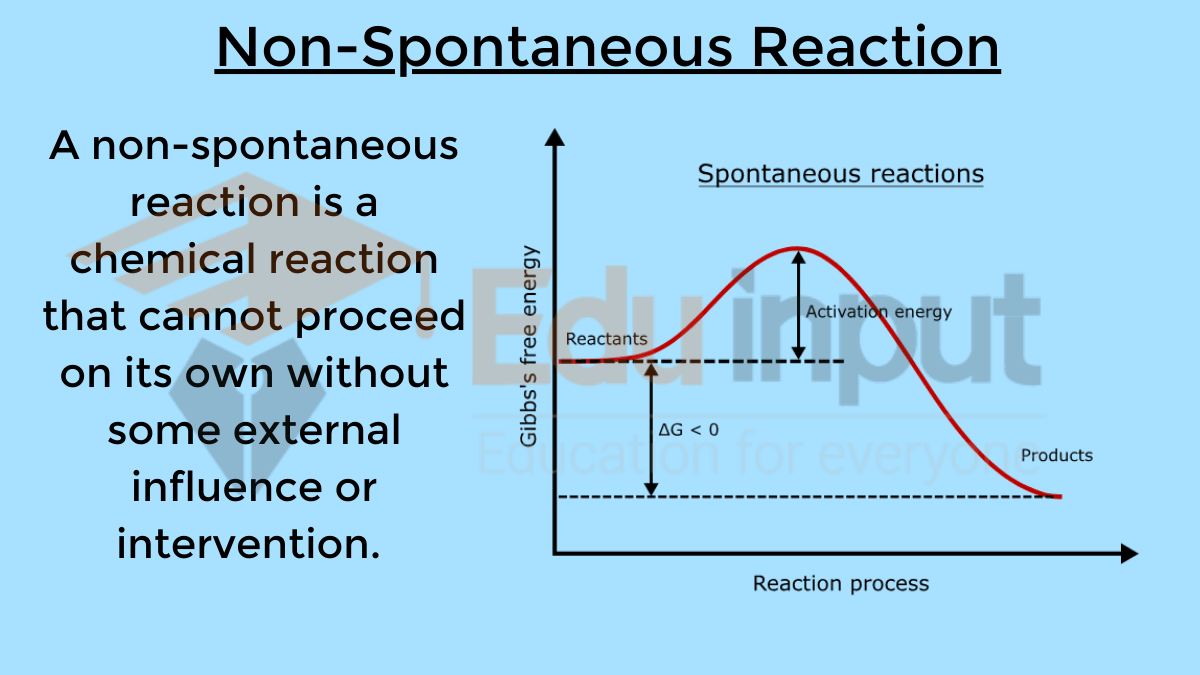
Why Endothermic Reactions Are Non-Spontaneous?
Endothermic reactions are often non-spontaneous because they do not occur naturally under standard conditions. Since they absorb heat from the surroundings, they need an external source of energy to initiate and sustain the reaction.
This energy input, known as the activation energy, is necessary to overcome the energy barrier that prevents the reaction from proceeding spontaneously.
Why Do Endothermic Reactions Feel Cold?
Endothermic reactions can feel cold to the touch because they draw heat energy from their surroundings.
When you come into contact with a system undergoing an endothermic reaction, the heat from your skin is transferred to the reaction, causing your skin to lose energy and feel cooler.
How to Identify Endothermic Reaction?
Identifying endothermic reactions involves observing certain key indicators like:
1. Temperature Decrease
A noticeable decrease in temperature in the reaction mixture or surroundings.
2. Positive ΔH
A positive change in enthalpy (ΔH) for the reaction, indicating heat absorption.
3. Energy Input
The reaction requires an external energy source or heating to occur.
Examples
Common examples of endothermic reactions include the melting of ice, the evaporation of liquids, the dissolution of certain salts in water (such as ammonium nitrate in cold packs), and chemical processes that absorb heat from their surroundings.



Leave a Reply