Osmium-Discovery, Properties, And Applications
Osmium is a chemical element with the atomic number 76 and the symbol Os. It is a hard, brittle, bluish-white transition metal that is the densest element known, with a density almost twice that of lead. Osmium has various industrial and scientific applications, including in electronics and as an alloying agent.
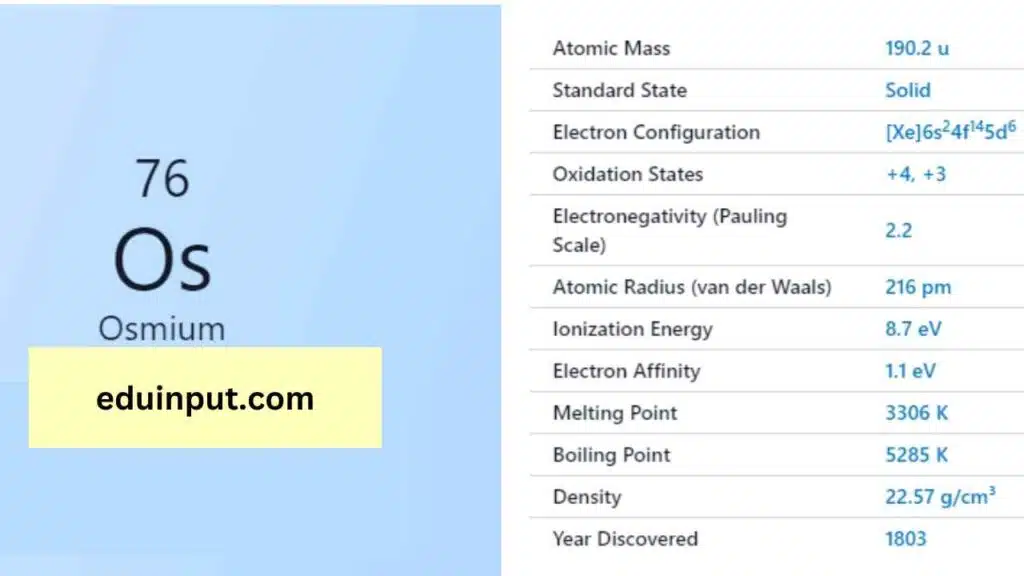
| Property | Value |
| Name | Osmium |
| Symbol | Os |
| Atomic number | 76 |
| Relative atomic mass (Ar) | Block in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Bluish grey |
| Classification | Metallic |
| Group in periodic table | 8 |
| Group name | Precious metal or Platinum group metal |
| Period in periodic table | 6 |
| Group in the periodic table | d |
| Shell structure | 2.8.18.32.14.2 |
| CAS Registry | 7440-04-2 |
Discovery
Osmium was discovered in 1803 by the English chemist Smithson Tennant while he was analyzing the residue of crude platinum. He found a black powder that he named “osmium,” derived from the Greek word for “smell,” because the compound had a strong odor.
Physical Properties
Osmium is a very dense, hard, and brittle metal with a bluish-white color. It has a very high melting point of 3,033°C, making it one of the most refractory elements. Osmium has a very low electrical resistance and is a good conductor of heat and electricity. Osmium is also highly resistant to corrosion and oxidation, making it useful in various applications.
Chemical Properties
Osmium is a transition metal that is highly reactive and forms many chemical compounds. It is resistant to corrosion and oxidation, and it has the highest oxidation state of any element. Osmium is also highly toxic and can be dangerous if not handled properly.
Facts
- Osmium is a hard, brittle, bluish-white transition metal that is the densest element known.
- Osmium was discovered in 1803 by the English chemist Smithson Tennant.
- Osmium is highly resistant to corrosion and oxidation and is a good conductor of heat and electricity.
Applications
Osmium has various industrial and scientific applications, including:
- Electronics: Osmium is used in the production of electrical contacts, which require high conductivity and resistance to corrosion and wear.
- Alloys: Osmium is used as an alloying agent in the production of specialty alloys, such as osmiridium, which is used in the manufacture of instrument pivots, phonograph needles, and electrical contacts.
- Scientific research: Osmium is used as a stain in biological research and as a marker in geology.
Osmium is a rare and valuable element with various industrial and scientific applications. Its high density, hardness, and resistance to corrosion and oxidation make it useful in electronics and as an alloying agent. Osmium is also used in scientific research as a stain and marker. However, due to its toxicity, it must be handled with care.




Leave a Reply