Germanium-Discovery, Properties, And Applications
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group. Germanium is a very important semiconductor material, commonly used in electronic and optical devices.
It is also used in infrared optics and in the polymerization catalyst industry. Germanium has interesting physical and chemical properties that make it a useful element in various applications.
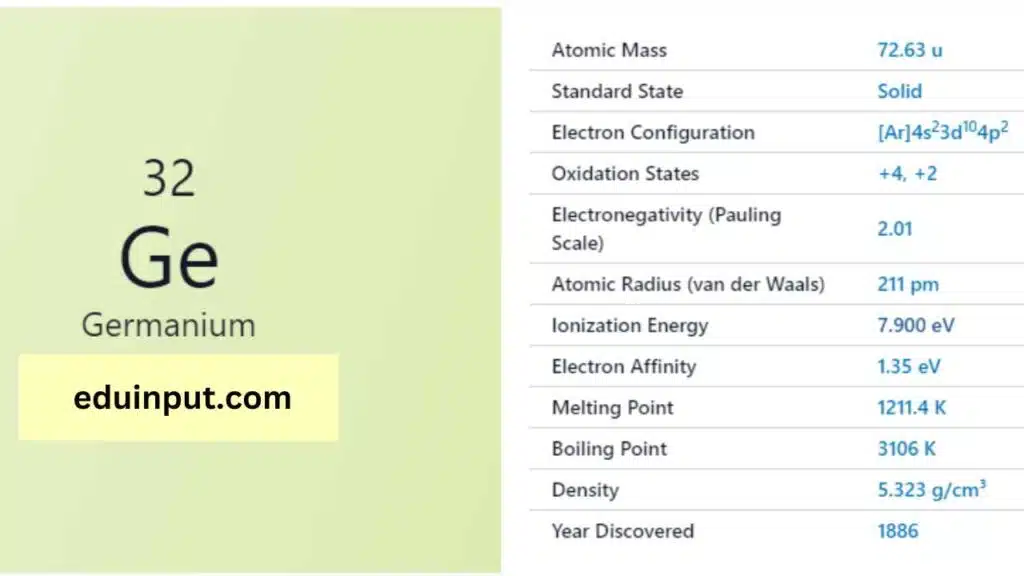
| Property | Value |
| Name | Germanium |
| Symbol | Ge |
| Atomic number | 32 |
| Relative atomic mass (Ar) | 72.630 |
| Standard state | Solid at 298 K |
| Appearance | Greyish white |
| Classification | Semi-metallic |
| Period in the periodic table | 14 |
| Group name | (none) |
| Block in the periodic table | 4 |
| Block in periodic table | p |
| Shell structure | 2.8.18.4 |
| CAS Registry | 7440-56-4 |
Discovery
Germanium was discovered in 1886 by the German chemist Clemens Winkler. Winkler discovered the element in a mineral called argyrodite, which is a silver sulfide mineral. Winkler named the new element Germanium after his homeland, Germany.
Physical Properties
Germanium is a metalloid, which means it has properties of both metals and nonmetals. It is a hard, grayish-white metal that is brittle at low temperatures. Germanium has a density of 5.323 g/cm3, which is less than that of iron. Its melting point is 938.3 K (665.2 °C), and its boiling point is 2833 K (2560 °C). Germanium is a semiconductor, which means it can conduct electricity under certain conditions.
Chemical Properties
Germanium is a relatively inert element and does not react with air, water, or dilute acids. However, it does react with concentrated nitric acid and hot concentrated sulfuric acid. Germanium forms compounds with oxygen, sulfur, and halogens. It also forms alloys with other metals, including iron, copper, and silver.
Facts
- Germanium is used in the manufacture of semiconductors, infrared optics, and polymerization catalysts.
- Germanium is found in small quantities in some ores, such as zinc ores and coal.
- Germanium is a rare element, making up only about 0.0005% of the Earth’s crust.
- Germanium has four stable isotopes and 26 radioactive isotopes.
- Germanium was the first element discovered by spectroscopy.
Applications
Germanium has several important applications in industry, science, and technology. Some of its applications are:
- Semiconductors: Germanium is an important semiconductor material, used in the manufacture of transistors, diodes, and other electronic devices. It is a key material in the development of computer chips and other microelectronic devices.
- Infrared optics: Germanium is transparent to infrared radiation, making it a useful material in infrared optics. It is used in the manufacture of lenses and windows for infrared spectroscopy and thermal imaging.
- Polymerization catalysts: Germanium compounds are used as catalysts in the production of certain types of plastics, such as polyethylene terephthalate (PET). Germanium-based catalysts are also used in the production of polypropylene and polybutene.
- Radiation detectors: Germanium is used as a radiation detector in nuclear physics experiments. It is also used in X-ray spectroscopy and gamma-ray spectrometry.
- Other applications: Germanium is used in the manufacture of solar cells, fiber optics, and other optical devices. It is also used in the production of alloys, such as brass and bronze.





Leave a Reply