Phosphorus-Discovery, Properties, And Applications
Phosphorus is a non-metallic element with the atomic number 15 and symbol P in the periodic table. It is a highly reactive element and is essential for life. Phosphorus exists in several allotropic forms, including white, red, and black phosphorus. The element is commonly used in fertilizers, detergents, and other industrial applications.

| Property | Value |
| Name | Phosphorus |
| Symbol | P |
| Atomic number | 15 |
| Relative atomic mass (Ar) | Colorless/Red/Silvery white |
| Standard state | Solid at 298 K |
| Appearance | Group in the periodic table |
| Classification | Non-metallic |
| Period in the periodic table | 15 |
| Group name | Pnictogen |
| Block in the periodic table | 3 |
| Block in periodic table | p |
| Shell structure | 2.8.5 |
| CAS Registry | 7723-14-0 |
Discovery
Phosphorus was discovered in 1669 by a German alchemist named Hennig Brand. He extracted it from urine by boiling it down and evaporating the liquid to a thick paste, which he then heated to a high temperature until it emitted a white glow. This substance was later identified as phosphorus.
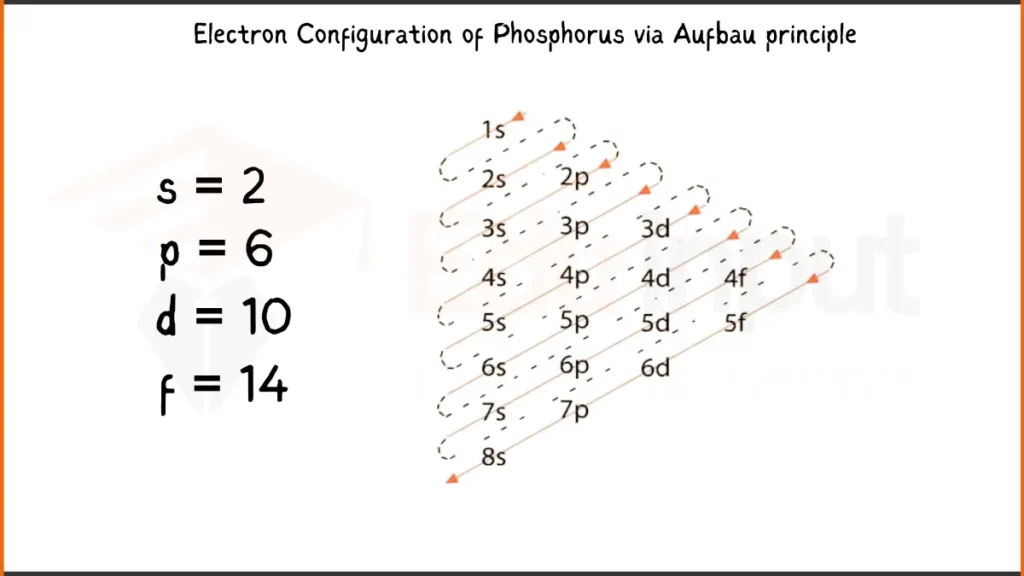
Electronic Configuration of Phosphorus
Phosphorus, has 15 electrons configured as 1s²2s²2p⁶3s²3p³. Like Sulfur, it has 2 electrons each in the first two shells, 6 in the next, and 2 in the third. However, Phosphorus has 3 instead of 4 in the outermost shell.
Electronic Configuration of Phosphorus via Bohr Model

Electronic Configuration of Phosphorus via Aufbau Principle
Physical Properties
Phosphorus is a non-metal with a pale yellow, waxy appearance. It has a melting point of 44.2 °C and a boiling point of 280 °C. It is a relatively soft element and can be cut with a knife. The element is insoluble in water but soluble in carbon disulfide.
Chemical Properties
Phosphorus is a highly reactive element, and it readily combines with other elements to form compounds. It reacts with oxygen to form phosphorus oxide and with sulfur to form phosphorus sulfide. Phosphorus also forms compounds with halogens, such as phosphorus trichloride and phosphorus pentachloride. It is also an essential element for living organisms, as it is a key component of DNA and RNA.
Facts
Phosphorus is the 11th most abundant element in the earth’s crust. -It is the second most abundant element in the human body after calcium. -The name “phosphorus” comes from the Greek word “phosphoros,” meaning “light-bringer,” because it glows in the dark.
Applications
Phosphorus is used in a variety of industrial applications. The majority of phosphorus is used in fertilizers to help plants grow. It is also used in detergents, water treatment, and the production of steel and other metals. Additionally, phosphorus is used in the production of flame retardants, semiconductors, and fireworks. In the medical field, phosphorus is used in bone replacement therapy and as a diagnostic tool in nuclear medicine.







Leave a Reply