Iron-Discovery, Properties, And Applications
Iron, symbolized as ‘Fe’ and occupying the atomic number 26, is a fundamental chemical element that has played a pivotal role in human history and continues to shape our modern world. This article explores iron’s essential properties, historical significance, and its diverse range of applications.
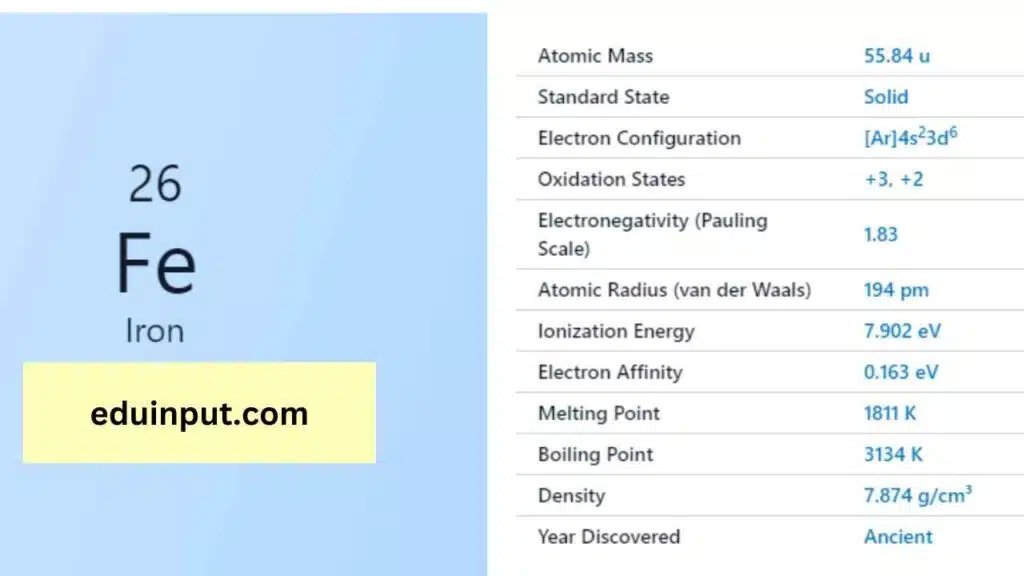
| Property | Value |
|---|---|
| Name | Iron |
| Symbol | Fe |
| Atomic number | 26 |
| Relative atomic mass (Ar) | 55.845 |
| Period in the periodic table | 4 |
| Group in the periodic table | 8 (Transition metal) |
| Block in the periodic table | d |
| Shell structure | 2.8.14.2 |
Discovery
Iron is one of the oldest known elements to humanity, with its use dating back to ancient civilizations. It was crucial in the development of various cultures, including the production of tools, weapons, and architectural marvels. The Iron Age, a period characterized by the widespread use of iron tools, marked a significant advancement in human history.
Physical Properties
Iron is a lustrous, silvery-gray metal that is solid at room temperature. It is renowned for its remarkable strength and durability. Iron has a melting point of 1,538°C and a boiling point of 2,861°C. It is a ferromagnetic material, meaning it can be magnetized.
Chemical Properties
Iron has numerous chemical applications and can readily form compounds with other elements. Its most common oxidation states are +2 and +3, which are essential in a variety of chemical reactions, including the rusting of iron when exposed to oxygen and water.
Facts
- Iron has four stable isotopes: Fe-54, Fe-56, Fe-57, and Fe-58, with Fe-56 being the most abundant.
- Hemoglobin, a protein in red blood cells, contains iron and is responsible for transporting oxygen throughout the body.
- Iron is a key component of steel, which is widely used in construction, transportation, and countless industrial applications.
Applications
Iron is a fundamental building block of modern society with diverse applications:
- Construction: Iron is a primary component in the construction industry, used in structures, bridges, and skyscrapers due to its strength and malleability.
- Transportation: Iron and steel are vital in the automotive and aerospace industries, where they provide strength and durability.
- Machinery: Iron is used in the production of machinery and equipment, as it can withstand high stress and wear.
- Medicine: Iron is crucial for human health as an essential element in hemoglobin, and iron supplements are often prescribed to treat iron-deficiency anemia.






Leave a Reply