Mercury- Discovery, Properties, And Applications
Mercury, represented by the symbol ‘Hg’ and possessing the atomic number 80, is a unique chemical element, known for its distinctive properties and historical significance.
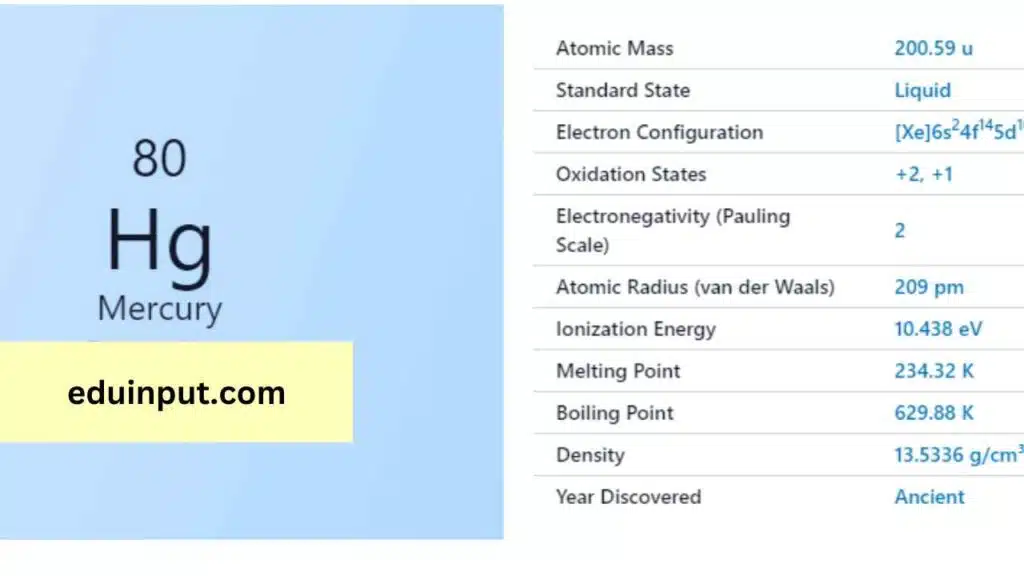
| Property | Value |
|---|---|
| Name | Mercury |
| Symbol | Hg |
| Atomic number | 80 |
| Relative atomic mass (Ar) | 200.59 |
| Period in the periodic table | 6 |
| Group in the periodic table | 12 (Transition metal) |
| Block in the periodic table | d |
| Shell structure | 2.8.18.32.18.2 |
Discovery
Mercury’s use dates back to ancient civilizations, including the Egyptians, who used it in cosmetics and the Chinese, who believed it had medicinal properties. Its name is derived from the Roman god Mercury, symbolizing speed and mobility.
Physical Properties
Mercury is the only metal that is liquid at room temperature. It has a shiny, silver-white appearance, with a density of approximately 13.6 g/cm³. Mercury has a melting point of -38.83°C and a boiling point of 356.73°C. It is a poor conductor of heat compared to other metals.
Chemical Properties
Mercury is relatively unreactive and resists oxidation. It forms amalgams with other metals, meaning it can dissolve them, forming alloys. However, it can become toxic in its elemental form, posing health risks if improperly handled.
Electron Configuration of Mercury
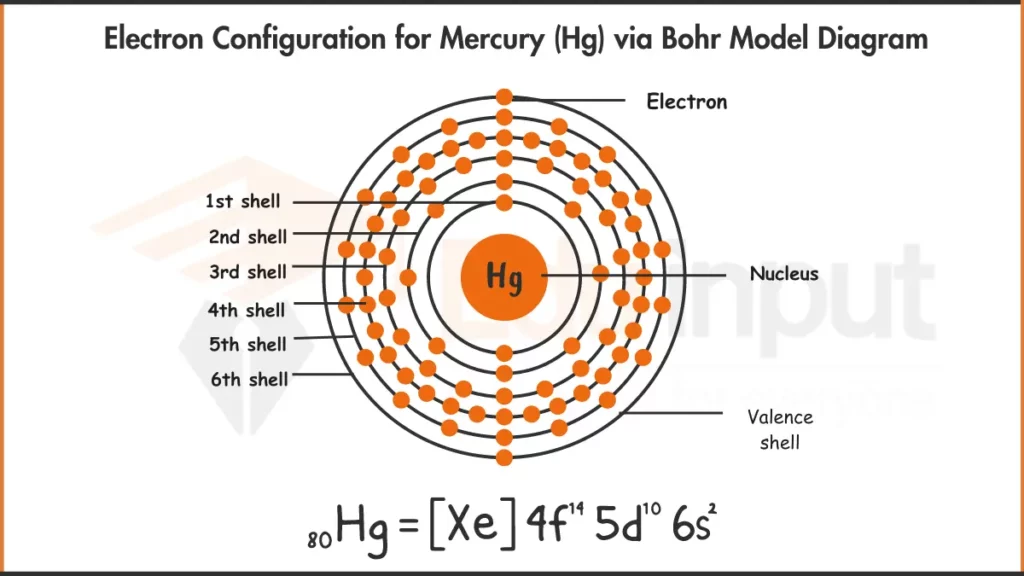
The electronic configuration of mercury is [Xe] 4f¹⁴ 5d¹⁰ 6s². It indicates a filled d-orbital subshell contributing to its stability.
Electron Configuration of Mercury via Bohr Model
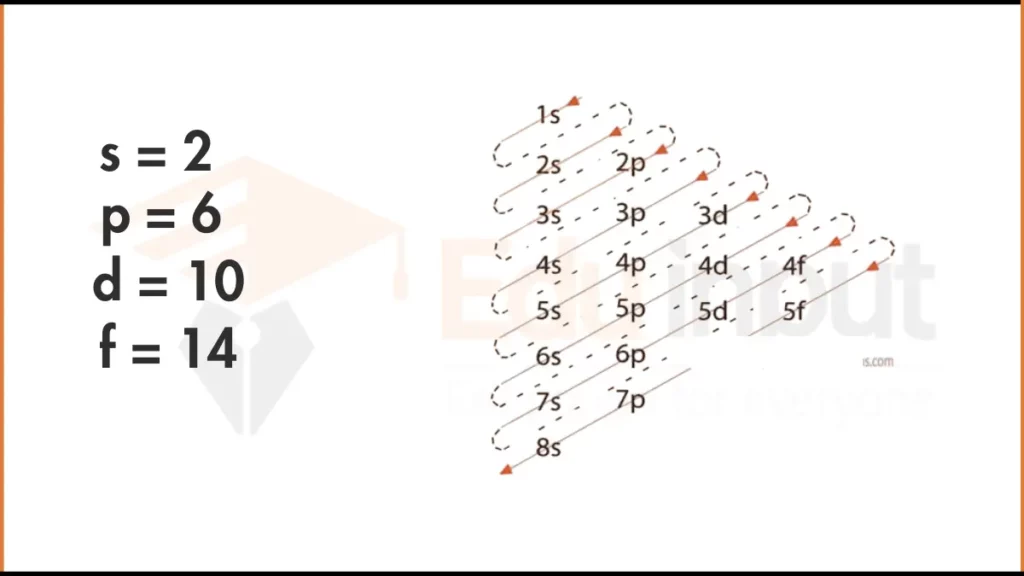
Electron Configuration of Mercury via Aufbau Principle
Facts
- Mercury is one of the few elements that are liquid at standard temperature and pressure.
- The toxic nature of mercury has led to stricter regulations on its use, especially in products like thermometers.
- It has had historical applications in various fields, including thermometers, barometers, and dental amalgams.
Applications
While the use of mercury has decreased due to its toxicity, it was historically employed in a range of applications:
- Thermometers: Mercury thermometers were widely used for measuring temperature until environmental concerns led to their replacement with safer alternatives.
- Barometers: Mercury barometers measure atmospheric pressure and have been critical in meteorology and weather forecasting.
- Dental Amalgams: Mercury-containing dental amalgams were used for dental fillings due to their durability and ease of use.
- Electricity Switches: Mercury switches were used in electrical applications for their reliability in making or breaking electrical connections.







Leave a Reply