Bond length, definition, trend, factors and applications
Definition
Bond length is the average distance between the nuclei of two bonded atoms in a molecule. It is a fundamental parameter that characterizes the size of a chemical bond and provides information about the three-dimensional arrangement of atoms within a molecule. The bond length is typically measured in angstroms (Å) or picometers (pm) and represents the equilibrium distance at which the attractive and repulsive forces between the atomic nuclei and electrons are balanced, resulting in a stable molecule.
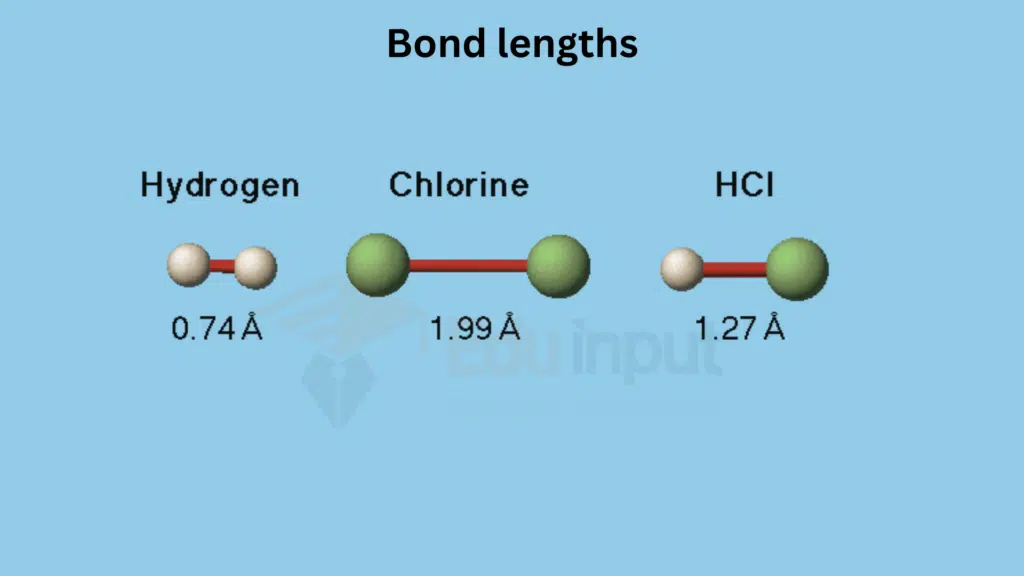
Introduction
Bond length is a fundamental concept in chemistry that refers to the average distance between the nuclei of two bonded atoms. This crucial parameter plays a significant role in shaping the molecular geometry and influencing the properties of substances. In this article, we will delve into the definition, importance, measurement, trends, factors affecting, and applications of bond length.
What is Bond Length?
At its core, bond length is the separation between the centers of two atoms that are covalently bonded. It is an essential factor in determining the three-dimensional arrangement of atoms in a molecule.
Bond length is a vital parameter that governs the geometry and behavior of molecules. Its influence on molecular structure, reactivity, and material properties makes it a key focus in the study of chemistry.
Why is Bond Length Important?
The importance of bond length lies in its direct correlation with the stability and reactivity of molecules. Understanding bond length provides valuable insights into the geometry of molecules, the type of bonds formed, and the overall behavior of substances in chemical reactions.
How is Bond Length Measured?
Experimental techniques such as X-ray crystallography and spectroscopy are commonly employed to measure bond lengths. X-ray crystallography involves analyzing the diffraction patterns of X-rays passing through a crystal to determine the positions of atoms accurately. Spectroscopic methods, including infrared and microwave spectroscopy, provide information about bond vibrations, allowing scientists to infer bond lengths.
Measuring Bond Length in Hydrogen Fluoride (HF) Using X-ray Crystallography
Formation of Single Crystals
Single crystals of hydrogen fluoride are prepared for analysis. The crystal must be of high quality and well-ordered to produce clear diffraction patterns.
Exposure to X-rays
X-rays are directed at the single crystal. The X-rays interact with the electron clouds surrounding the atoms in the crystal, causing them to diffract.
Diffraction Pattern
The diffracted X-rays create a diffraction pattern, which is captured on a detector. The pattern is a series of spots corresponding to the arrangement of atoms in the crystal.
Analysis of Diffraction Pattern
The diffraction pattern is analyzed to determine the angles and intensities of the diffracted X-rays. Mathematical algorithms are then applied to convert this information into an electron density map.
Construction of Electron Density Map
The electron density map shows the distribution of electrons within the crystal. By interpreting this map, the positions of the nuclei in the molecule can be determined.
Bond Length Determination
The distance between the nuclei of the bonded atoms in the molecule corresponds to the bond length. In the case of hydrogen fluoride (HF), the bond length is the distance between the hydrogen and fluorine nuclei.
Simplified Result
The X-ray crystallography analysis of hydrogen fluoride might reveal a bond length of approximately 0.92 angstroms (Å).
Trends in Bond Length
Bond Length and Atomic Radius
There is a general trend: as the atomic radius increases, the bond length also increases. Larger atoms have electrons in orbitals that extend farther from the nucleus, resulting in longer bonds.
Bond Length and Bond Order
Bond order, indicating the number of bonds between two atoms, influences bond length. As bond order increases (e.g., single to double to triple bonds), bond length decreases.
Bond Length and Electronegativity
Electronegativity differences between atoms affect bond length. Higher electronegativity differences lead to shorter bond lengths due to increased electron density towards the more electronegative atom.
Bond Length and Hybridization
The type of hybridization of atomic orbitals affects bond length. For example, sp³ hybridization generally leads to longer bonds compared to sp² or sp hybridization.
Factors that Affect Bond Length
Bond Type (Single, Double, Triple)
Generally, as the bond order increases (single to double to triple bonds), the bond length decreases. This is because more shared electrons result in a stronger attraction between nuclei.
Resonance
Resonance structures can influence bond length. The actual bond length is often considered as an average of the bond lengths in different resonance structures.
Steric Effects
Large groups or lone pairs can cause steric hindrance, affecting bond length. Repulsion between electron clouds may lead to longer bonds.
Intermolecular Interactions
Interactions between molecules, such as hydrogen bonding, can influence bond length. These interactions may cause deviations from the expected bond lengths.
Bond Length and Bond Strength
Relationship Between Bond Length and Bond Strength
Generally, shorter bonds are stronger. As the distance between nuclei decreases, the attraction between the positively charged nuclei and negatively charged electrons increases, leading to a stronger bond.
Exceptions to the General Trend
Some examples of chemical compounds exhibit deviations from the expected bond length trends due to factors like resonance, steric effects, and unique electronic configurations.
Applications of Bond Length
Predicting the Structure of Molecules
Bond length information helps predict the three-dimensional structures of molecules, which is crucial for understanding molecular behavior.
Determining the Type of Bond Between Atoms
Different bond lengths are associated with different types of bonds. Longer bonds are typically indicative of weaker single bonds, while shorter bonds often correspond to stronger double or triple bonds.
Understanding the Properties of Materials
Knowledge of bond lengths is essential for designing and understanding the properties of materials. For example, in polymers, bond lengths influence mechanical strength and flexibility.
What is bond length in a molecule?
Bond length is the average distance between the nuclei of two bonded atoms within a molecule. It is a fundamental parameter that defines the size of a chemical bond.
How is bond length measured experimentally?
Bond length is measured using experimental techniques such as X-ray crystallography and spectroscopy. X-ray crystallography involves analyzing the diffraction patterns of X-rays passing through a crystal, while spectroscopy provides information about bond vibrations.
Why is bond length important in chemistry?
Bond length is crucial because it influences the three-dimensional structure of molecules, impacting their properties, reactivity, and behavior in chemical reactions. It provides valuable insights into the nature of chemical bonds.
What factors affect bond length in a molecule?
Several factors influence bond length, including the types of atoms involved, bond order (single, double, or triple bonds), electronegativity differences, hybridization of atomic orbitals, and the presence of resonance structures.
How does bond length relate to bond strength?
Generally, shorter bonds are stronger. As the distance between nuclei decreases, the attraction between the positively charged nuclei and negatively charged electrons increases, resulting in a stronger bond. However, there can be exceptions based on factors like resonance and steric effects.
Do all bonds in a molecule have the same bond length?
No, not all bonds in a molecule have the same bond length. The bond length depends on various factors, including the types of atoms involved, the presence of multiple bonds, and the influence of resonance structures. Different bonds within a molecule can exhibit different lengths.
Can bond length be used to predict the properties of materials?
Yes, bond length is an important parameter in predicting the properties of materials. It influences factors such as mechanical strength, flexibility, and conductivity in materials like polymers and metals. Understanding bond length aids in designing materials with specific characteristics.
How does bond length contribute to the stability of molecules?
The bond length is directly related to the stability of molecules. Shorter bonds generally indicate stronger bonds and contribute to the overall stability of the molecule. Knowledge of bond length is crucial in predicting the stability of different molecular structures.






Leave a Reply