RULES FOR THE DISTRIBUTION OF ELECTRONS
There are three important rules for electrons in subshells and orbitals of atoms:
(1) Auf- Bau Principle
(2) Paul’s Exclusion Principle
(3) Hund’s Rule
Auf- Bau Principle
The electron should be filled in energy subshells in the order of increasing energy values. It means that the electron should be first placed in 1s sub- shell (orbital), then in 2s, then 2p, and so on.
Paul’s Exclusion Principle
It is defined in two ways:
(i) The two electrons in the same orbital should have opposite spins.
(ii) No two electrons in an atom can have all four quantum numbers same.
Hund’s Rule
If degenerate orbitals are available and more than one electrons are to be placed in them, they should be placed in separate orbitals with the same spin rather than putting them in the same orbital with opposite spins.
Explanation: Suppose we have to place three outermost electrons in the 2p subshell of the nitrogen atom. They will be distributed as follows:
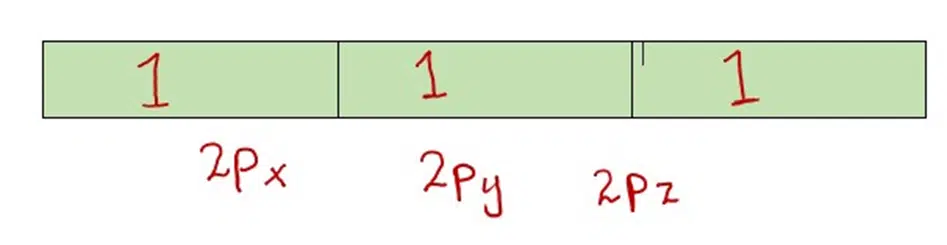
But not as
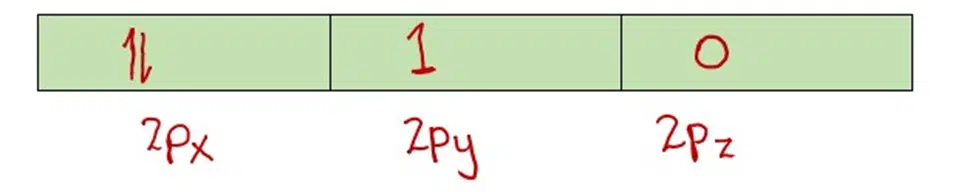
The whole electronic configuration revolves around these three rules. If you need to know in detail about these topics read our in-depth articles on respective rules.



Leave a Reply