Electron distribution in shells and sub-shells | Division of shells into orbitals
The maximum number of electrons that can be accommodated in any shell is given by the formula ‘2n2’
A shell is further divided into subshells.
| n= 1 | has only one subshell 1s |
| n= 2 | has only two subshells 2s, 2p |
| n= 3 | has only three subshells 3s, 3p, 3d |
| n= 4 | has only four subshells 4s, 4p, 4d, 4f |
| n= 5 | has again four subshells i.e. 5s, 5p, 5d, 5f |
Actually, there should have been five sub- shells in n= 5. The number of elements in the Periodic Table, which have been discovered up to this time is around 110. So we don’t need the fifth sub- shell for filling the electrons in any of the atoms. n= 5 has again four sub- shell i.e. 6s, 6p, 6d, 6f.
Division of subshells into orbitals
Subshells are further subdivided into orbitals. These orbitals are named s, p, d, and f.
s subshell has only one orbital.
p subshell has three orbital. i.e. px, py, and pz.
d subshell has five orbitals dxy, dyz, dzx,dz2,dx2– y2
f subshell has seven orbitals.
Three orbitals of p subshells are triply degenerate.
Five orbitals of p subshells are five times degenerate.
Seven orbitals of f subshells are seven times degenerate.
The maximum number of electrons in ‘p’ subshells is six.
The maximum number of electrons in ‘d’ subshells is ten.
The maximum number of electrons in ‘f’ subshells ate fourteen.
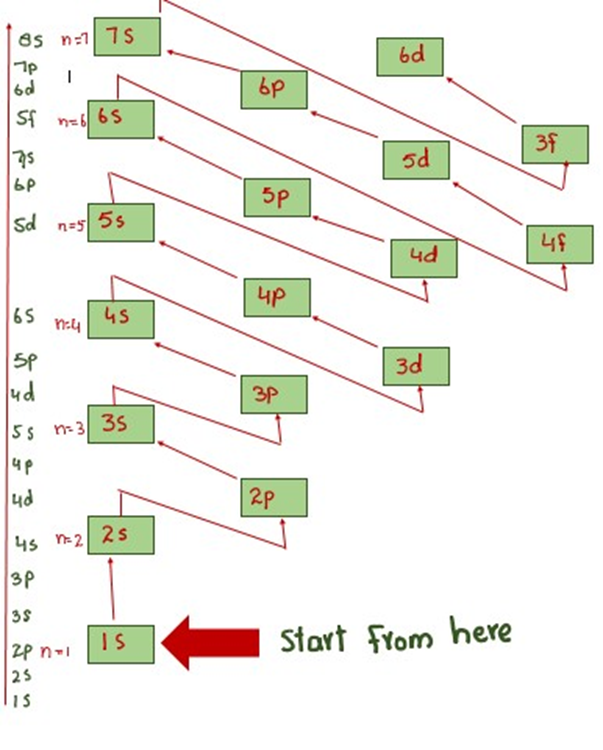
The arrangement of the orbitals keeping in view the energy values and principle quantum number is as follows.

What is (n+ ℓ) RULE
The arrangement of subshells that have been done previously needs modification. Actually, the arrangement of the sub-shells should involve azimuthal quantum numbers as well. We so the addition of principle and azimuthal quantum number.
Definition of (n+ ℓ) RULE
The orbitals are arranged in the increasing order of (n+ ℓ) values. If any two orbitals have the same (n+ ℓ) values, then that orbital should be placed first whose ‘n’ value is smaller.
Table of (n+ ℓ) values

So energy-wise arrangement, which is followed for electronic configuration is as followed in below fig.
This arrangement is shown in below fig of subshell can also be achieved by the diagram.



Leave a Reply