Nuclear Physics-Definition And Applications
Nuclear Physics is the study of subatomic particles that makes up the nucleus of all matter. It is mainly concerned with the properties of nuclear materials, processes, and nuclear reactions.
Nuclear Physics
It is the study of atomic nuclei. As a result, it is important to have a thorough understanding of nuclear physics. Theoretical physicists explore these phenomena while experimental physicists observe and measure the results. There are some basic forces of nature nuclear force is one of them.
It is the study of Atomic Nuclei. Isotopes are nuclei that have the same charge number but a different mass number. The mass of the isotopes is determined by a mass spectrograph.
The results of experiments on the masses of different nuclei show that the mass of the nucleus is always less than the total mass of all the protons and neutrons making up the nucleus.
In the nucleus missing mass is called the mass defect. It has been observed that those elements whose charge number is greater than 82 are unstable. Such elements are called radioactive and the phenomenon is called radioactivity.
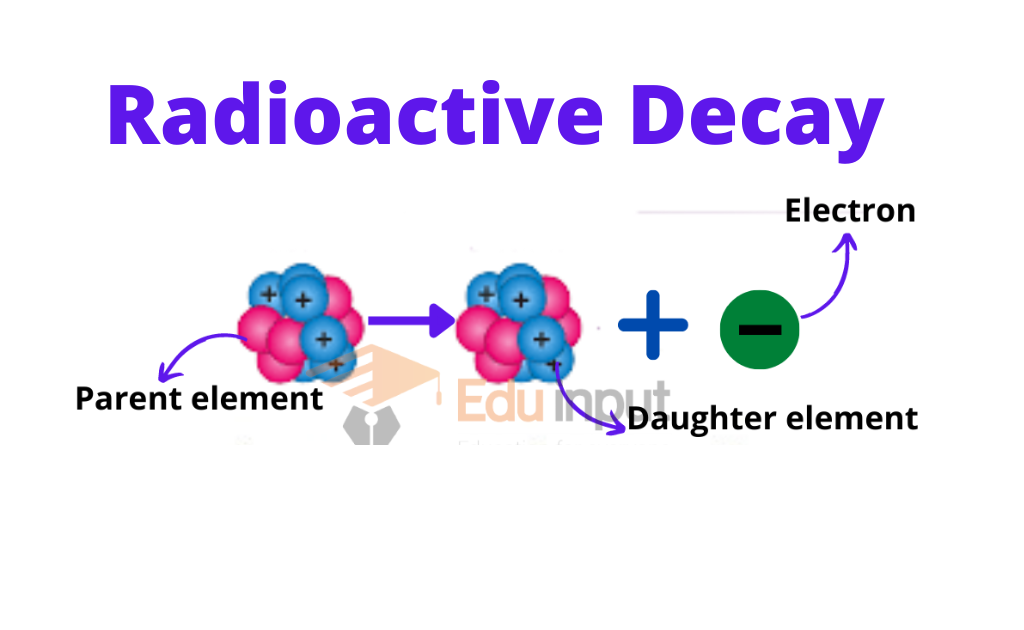
During radioactivity decay radiations are produced. During nuclear change, the law of conservation of momentum, mass, and energy remains applicable.
The half-life of a radioactive element is that period in which half of the atom decays. During decay, radiations are produced. These radiations interact with matters and ionize them. Radiations can be detected by radiation detectors. Wilson cloud chamber and Geiger Muller counter are the main radiation detectors. Exposure to radiation is harmful to humans. There are biological effects of radiation. Radiations are also useful in different fields. e.g. tracer techniques.
Nuclear physics also studies nuclear reactions. There are many types of nuclear reactions e.g. nuclear fission, fission chain reaction, and fusion reactions. In nuclear reactors, fission chain reactions take place to produce an electric current.
Difference between Nuclear Chemistry and Nuclear Physics
It is the study of the structure, composition, and behavior of atoms and subatomic particles. The main topic of this branch is called nuclear physics, which is closely related to nuclear chemistry. Nuclear chemistry is the study of chemical reactions in the nucleus.
Difference between Atomic Physics and Nuclear Physics
The study of Nuclear Physics is based on the understanding of atomic nuclei, which is the core of all matter. Atoms are the smallest units of matter. While Atomic Physics is the study of atoms and the electronic configuration of electrons.
A proton is a positively charged particle, and a neutron is a neutral, negatively charged particle. The nucleus is the main part of an atom and the particles are called nucleons. Each nucleon is composed of a positively charged quark and a negatively charged antiquark.
Protons are composed of two up quarks and one down quark, while neutrons are composed of one up quark and two down quarks.
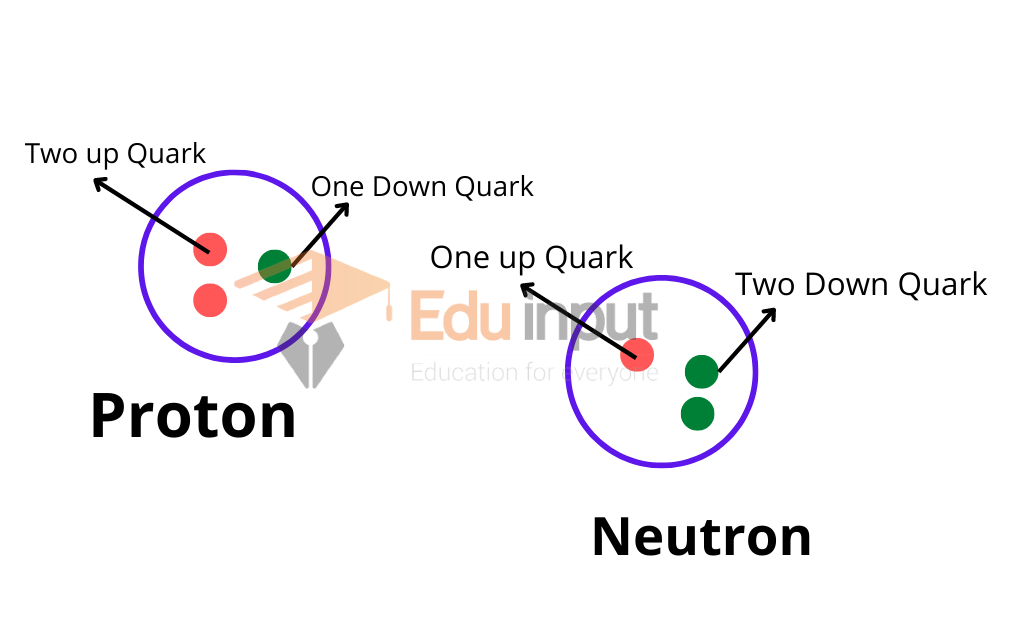
Nucleons form the nucleus of an atom and are responsible for holding the atom together.
Protons and neutrons are called baryons and anti-baryons, respectively. An anti-baryon is a particle with the opposite charge to that of a proton. There are three different types of anti-baryons: anti-proton, anti-neutron, and anti-electron.
Atoms are made up of electrons, protons, and neutrons. Electrons orbit the nucleus of an atom and are electrically neutral. Protons are much more massive than electrons and therefore move much faster. They carry positive charges and are attracted to each other by electric forces.
Nuclei are the most stable part of the atom. Due to their stability, the nuclei cannot be separated, which is why they make up the nucleus of the atom. Nuclei are the building blocks of the atom, and everything is composed of atoms.
Nuclei are surrounded by outer electron shells. These outer electron shells contain electrons, and they are the most loosely bound part of the atom.
These electrons are very mobile, and they carry a negative charge. The number of electrons in the outer electron shell varies from atom to atom.
All atoms consist of a positively charged nucleus and outer electrons. The number of electrons in the outer electron shells is determined by the number of protons in the nucleus.
Nature of Nuclear Force
The nuclear force is the weak force, and it functions as the gravitational force between particles of matter, such as electrons and protons.
The nuclear force is much stronger than the Coulomb force, which is why you don’t see it acting between the electrons and nuclei of atoms.
Nuclear physics is based on the forces known as nuclear forces. The nature of nuclear force is given below:
- The nuclear force is a force of attraction.
- It does not depend on charges.
- The nuclear force is a short-range force.
- When we decrease the distance between two nucleons, the nuclear force becomes weak between them.
- The nuclear force depends on the spin.
Application of Nuclear Physics
Discoveries in nuclear physics have brought about programs in lots of fields. These consist of nuclear energy, nuclear weapons, nuclear remedy and magnetic resonance imaging, commercial and agricultural isotopes, ion implantation in substances engineering, and radiocarbon relationship in geology and archaeology.
Related FAQs
Who is the father of nuclear physics?
Ernest Rutherford is known as the father of nuclear physics.
What is the use of nuclear physics?
Nuclear energy is a part of any society’s energy needs. Archaeologists have used a nuclear reactor to analyze the isotope compositions of ancient objects to determine the types of materials used to make them.
What are isotopes?
Isotopes are nuclei that have the same charge number but a different mass number.
What is anti-baryon?
An anti-baryon is a particle with the opposite charge to that of a proton.
How many types of anti-baryon?
There are three different types of anti-baryons:
anti-proton.
anti-neutron.
and anti-electron.

 written by
written by 




Leave a Reply