Beta Decay-Definition, Types, Application, And Limitations
Beta Decay Definition
Beta decay is a radioactive process where an atomic nucleus emits a beta ray, which transforms a proton into a neutron (β– decay) or a neutron into a proton (β+ decay).
This process occurs where an unstable atomic nucleus undergoes a transformation to attain a more stable configuration by emitting a beta particle. A beta particle can be either an electron (beta minus decay) or a positron (beta plus decay).
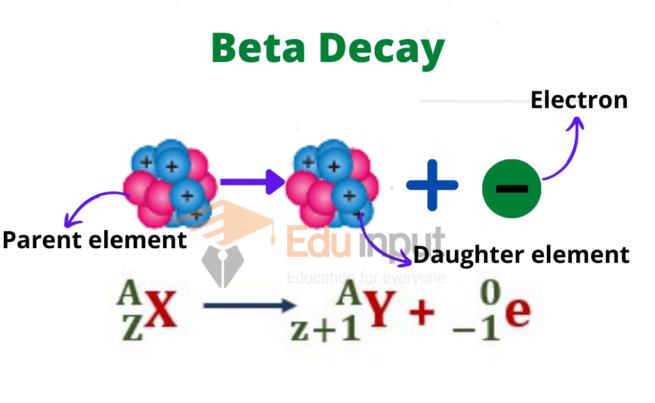
This emission occurs when the nucleus has an excess of either neutrons (beta minus decay) or protons (beta plus decay). The emission of a beta particle alters the atomic number of the nucleus, bringing it closer to stability.
The symbol used to represent beta decay is the Greek letter “β.” It can be written as a superscript to the left of the atomic symbol of the parent nucleus.
Beta Decay Equation
The formula for beta decay can be expressed as:
Parent Atomic Symbol-Mass ⟶ Daughter Atomic Symbol-Mass + β
The beta particle is represented by the symbol “β.” The atomic symbols represent the elements, and the masses represent the atomic masses of the parent and daughter nuclei.
For Example, Thorium 23490Th transforms into protactinium 23491Pa after the emission of the β-particle.

Types of Beta Decay
There are two types of beta decay.
- Beta Minus Decay
- Beta Plus Decay
Beta-minus Decay
Beta-minus decay occurs when a neutron in the nucleus converts into a proton, resulting in the emission of an electron (e-) and an antineutrino (ν̄e). This process is represented by the decay equation:
n ⟶ p + e- + ν̄e
Beta-plus Decay
In beta-plus decay, a proton within the nucleus transforms into a neutron, emitting a positron (e+) and a neutrino (νe). The decay equation for beta-plus decay can be written as:
p ⟶ n + e+ + νe
Role of Neutrinos in Beta Decay
Neutrinos play a crucial role in beta decay. During beta-minus decay, an antineutrino (ν̄e) is emitted, carrying away energy and momentum to ensure conservation laws are upheld. Similarly, in beta-plus decay, a neutrino (νe) is emitted to maintain the balance of energy and momentum within the system. These elusive particles contribute to the overall understanding of nuclear processes and offer insights into the nature of the weak nuclear force.
Applications of Beta Decay
Beta decay finds applications in a multitude of scientific and practical fields. Let’s explore some of its significant applications:
Radiocarbon Dating
Radiocarbon dating, a technique used to determine the age of organic materials, relies on the principle of beta decay. By measuring the decay rate of carbon-14 (14C) in a sample, scientists can estimate the time that has elapsed since the organism’s death.
This technique has revolutionized archaeology and paleontology, enabling researchers to establish chronologies and unravel the history of ancient civilizations.
Medical Imaging and Radiotherapy
In the realm of medicine, beta decay plays a pivotal role in both diagnostic imaging and radiotherapy. Radiotracers containing beta-emitting isotopes, such as technetium-99m, are employed in nuclear imaging techniques like Single-Photon Emission Computed Tomography (SPECT). These radioactive isotopes enable physicians to visualize and diagnose various medical conditions non-invasively.
Furthermore, beta radiation can be utilized for therapeutic purposes in cancer treatment. Radioactive isotopes, such as iodine-131, emit beta particles that target cancerous cells, effectively destroying them while minimizing damage to surrounding healthy tissues. This technique, known as targeted radionuclide therapy, has emerged as a valuable tool in the fight against cancer.
Nuclear Power Generation
Beta decay also has a significant impact on the field of nuclear power generation. Certain isotopes, such as uranium-235 and plutonium-239, undergo beta decay, releasing energy in the process.
This energy can be harnessed to generate electricity in nuclear power plants. Understanding the mechanisms of beta decay and the associated safety considerations is crucial for the development and maintenance of efficient and secure nuclear power facilities.
Limitations and Risks of Beta Decay
While beta decay has its scientific and technological merits, it is crucial to consider the limitations and potential risks associated with this phenomenon.
Radioactive Contamination
Beta-emitting radioactive isotopes can pose a risk of contamination if not handled properly. Accidental releases of radioactive materials, such as isotopes undergoing beta decay, can have detrimental effects on the environment and human health. Stringent safety measures and proper disposal methods are vital to mitigate these risks.
Health Risks
Exposure to beta radiation, particularly in high doses or over prolonged periods, can have adverse health effects. The ionizing nature of beta particles can damage biological tissues and DNA, leading to various health conditions, including radiation sickness and an increased risk of cancer. Strict adherence to radiation safety guidelines and regulations is paramount in minimizing these risks.

 written by
written by 



Leave a Reply