Determination of charge of electron by Millikan’s oil droplet method
In 1909, MILLIKAN determined the charge of the electron with the help of his designed apparatus.
Apparatus of Millikan’s oil drop experiment
It consists of a metallic chamber. It is divided into two parts. The upper parts are used for the storage of the oil droplets which are produced with the help of an atomizer.
The lower part of the electrical field. The upper electrode has a hole in it. This lower part of the chamber contains windows. One of the windows is used to illuminate the field of view. The other is used for the x-rays to ionize the gas inside the chamber.
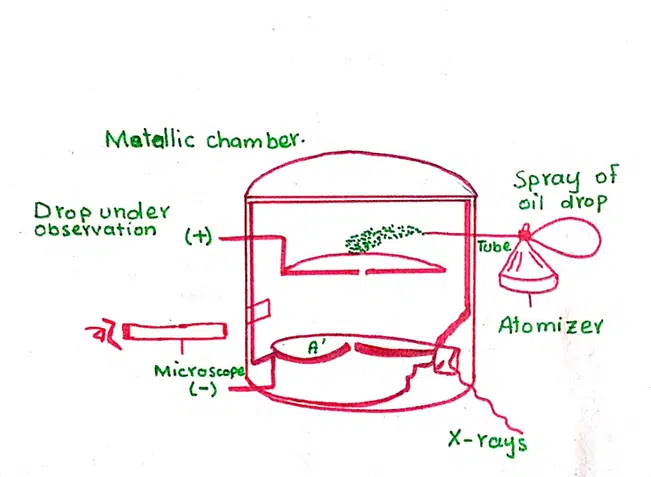
Working of the apparatus:
When the help of an atomizer a fine spray of a suitable oil is introduced into the upper chamber. A few drops are allowed to pass through hole H and enter the lower chamber. Then the hole is closed and the velocity of the drop with which it falls down is noted. The velocity ‘V1’ of the droplet is proportional to the weight of the droplet.
V1 ∝ mg –> (1)
With the help of x-rays, the air in the chamber is ionized. The drop, which is under observation, takes up a gaseous ion and gets charged. At this time, plates A and A’ are connected to the battery. The electrical field strength ‘E’ comes into action and the charged droplet moves upwards. Let the upward velocity be ‘v2’. The upward velocity is proportional to the net upward force.
This net force is a difference of upward electrical force minus the gravitational pull on electrons.
V ∝ eE – mg ———–(2)
Where Ee is the force on the electron due to the electrical field.
V1/v2 = mg/ Ee – mg –> (3)
The velocity ‘v1’ and ‘v2’ are noted by means of a telescope. ‘E’ is the strength of the electrical field, ‘g’ is the acceleration due to gravity, and ‘m’ the mass of the droplet. This mass can be calculated with the help of Stroke’s formula. In this way, the charge of the electron ‘e’ can be calculated.
Findings of Millikan’s Oil droplet Experiment
Millikan came to know that the charge on each drop was different. So he reached the conclusion that the smallest charge on any droplet is 2.59 x 10-19coulombs. The recent value is 1.602 x 10-19coulombs, and this is the charge of electrons. Actually, a droplet captures the electron.
Mass of electron:
By joining the results of the two experiments i.e. J.J Thomson experiment and Millikan, one can calculate the electron.
E = 1.602 x 10-19 C –> (4)
e/m = 1.758 x 1011 C kg-1
Putting (4) into (5)
m = 1.602 x 10-19C/ 1.758 x 1011 C kg -1 = 9.1095 X 10-31 kg
Properties of subatomic particles
The comparative study of electron, proton and neutron is given in the table.
| Charge | Mass in Kg | Mass in a.m.u | Charge | Discovery |
| Electron Proton Neutron | 9.108 x 10-31kg 1.672 x 10-27kg 1.672 x 10-27 kg | 0.005493 a.m.u 1.005757 a.m.u 1.00598 a.m.u | 1.602 x 10-19C 1.602 x 10-19C No charge | J.J Thomson J.J Thomson Chadwick |



Leave a Reply