What is an atomic model in chemistry?
An atomic model is a representation of the chemical elements. In a model, the electrons of atoms are arranged in a specific order. The model helps the person to understand the atom more clearly.
The atomic model shows the way in which the electrons move around the nucleus, the number of protons, and the number of neutrons. If you are a student then you must learn to make an atomic model of the elements that you want to learn.
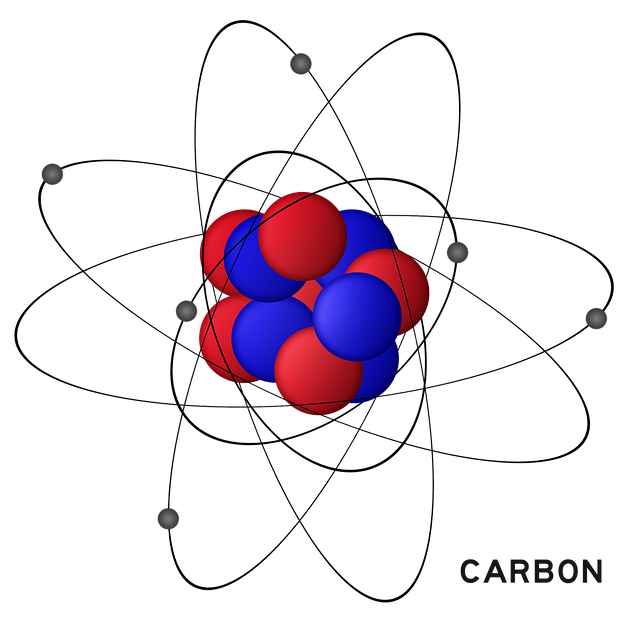
What does an atomic model look like?
An atomic model is a three-dimensional model that is made up of pieces of different shapes and colors. These models are based on the chemical element that is being studied.
Various experiments have told us that atoms are divisible. Once the divisibility of the atoms is accepted, we should come to know how the fundamental particles are arranged in an atom.
In designing an atomic model, we use all the available experiment observations. A model may be modified or replaced, by another model. This is due to the new experimental observation.
Three famous atomic models
Three famous atomic models of atomic structure are:
- Rutherford’s atomic model (1911).
- Bohr’s atomic model (1926).
- Quantum mechanical model of the atom (1926).
Conclusion
These are the different types of atomic models. Nowadays, students use these models to make presentations or they can also be used as a part of a project.
If you are a student and you are looking for the right place to learn then you must try to get the Atomic Model in Chemistry. The Atomic Models in Chemistry will be useful for you and will help you to make better presentations.



Leave a Reply