Difference between crystal lattice and lattice point
Crystal lattice and lattice point are two related concepts in crystallography. While both are essential in understanding the arrangement of atoms or molecules in a crystal, they are distinct from each other.
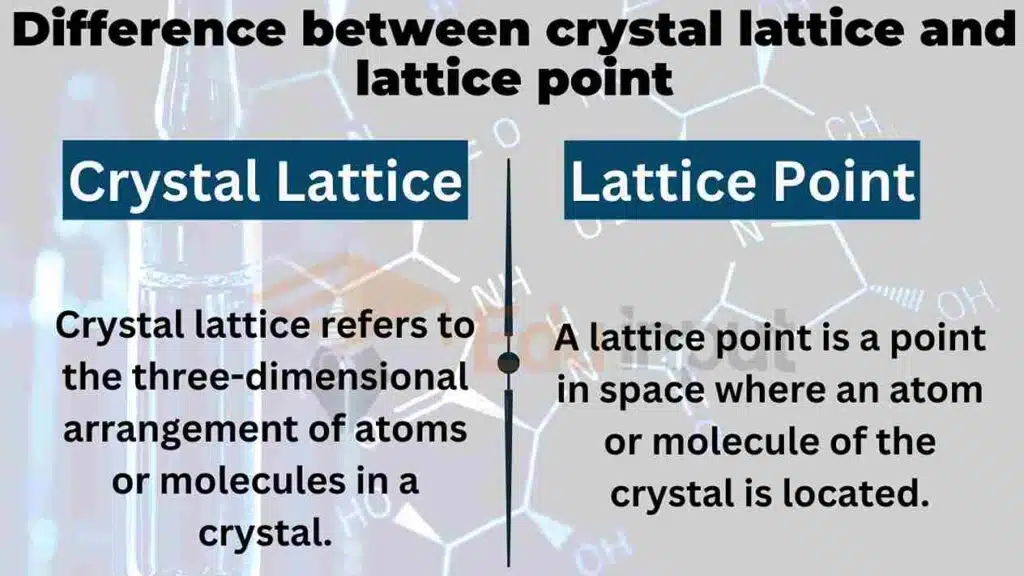
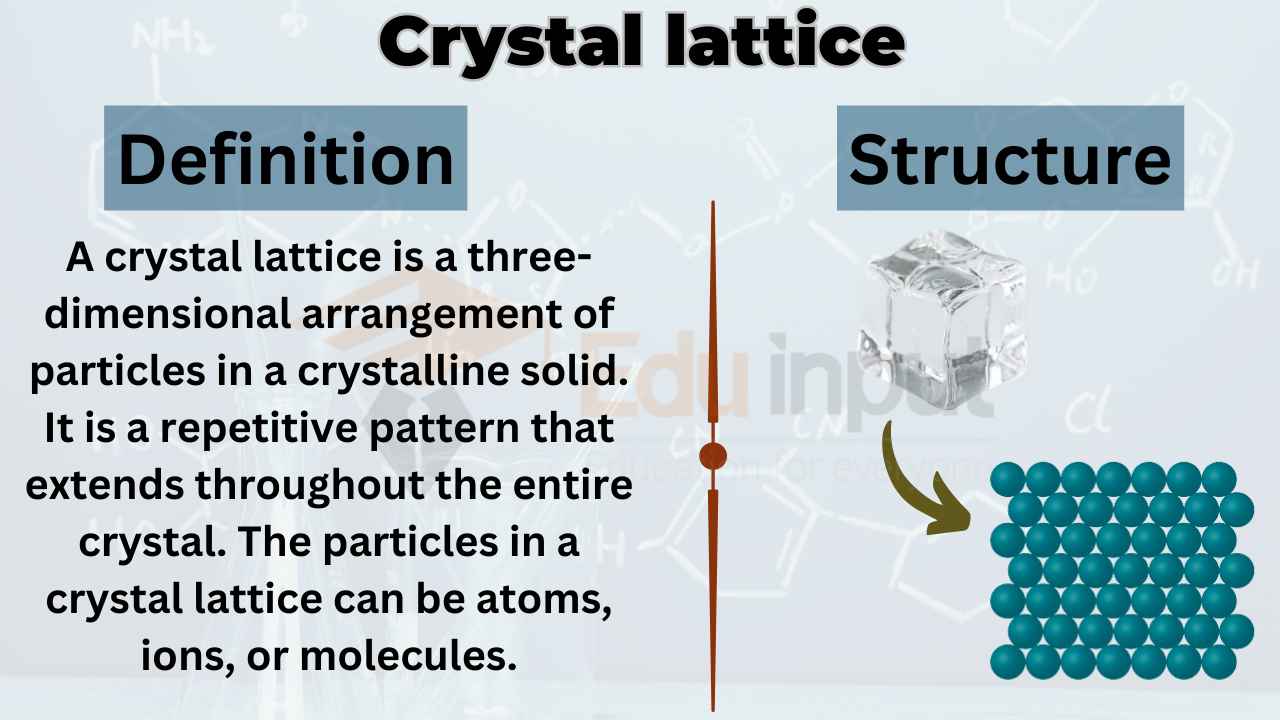
Crystal lattice refers to the three-dimensional arrangement of atoms or molecules in a crystal. It can be described as a repeating pattern of points in space, which defines the crystal’s geometry and symmetry.
A crystal lattice is characterized by its unit cell, which is the smallest repeating unit of the lattice. The unit cell can be visualized as a parallelepiped with edges defined by the crystallographic axes.
On the other hand, a lattice point is a point in space where an atom or molecule of the crystal is located. It is the intersection of the lattice lines, which connect the neighboring unit cells. In other words, a lattice point is a position in the crystal lattice where an atom or molecule can be found.
Difference between crystal lattice and lattice point
The difference between crystal lattice and lattice point can be better understood with the following examples:
Example 1: Sodium chloride crystal
The crystal lattice of sodium chloride is face-centered cubic (FCC), which can be visualized as a cube with sodium ions at the corners and chlorine ions at the face centers. The unit cell of sodium chloride is also cubic and contains one sodium ion and one chlorine ion.

The lattice points in sodium chloride crystals are the positions where the sodium and chlorine ions are located. These lattice points form a three-dimensional array of points that define the crystal lattice.
Example 2: Diamond crystal
The crystal lattice of a diamond is a face-centered cubic lattice with each carbon atom covalently bonded to four neighboring carbon atoms. The unit cell of a diamond is also a cube and contains eight carbon atoms.
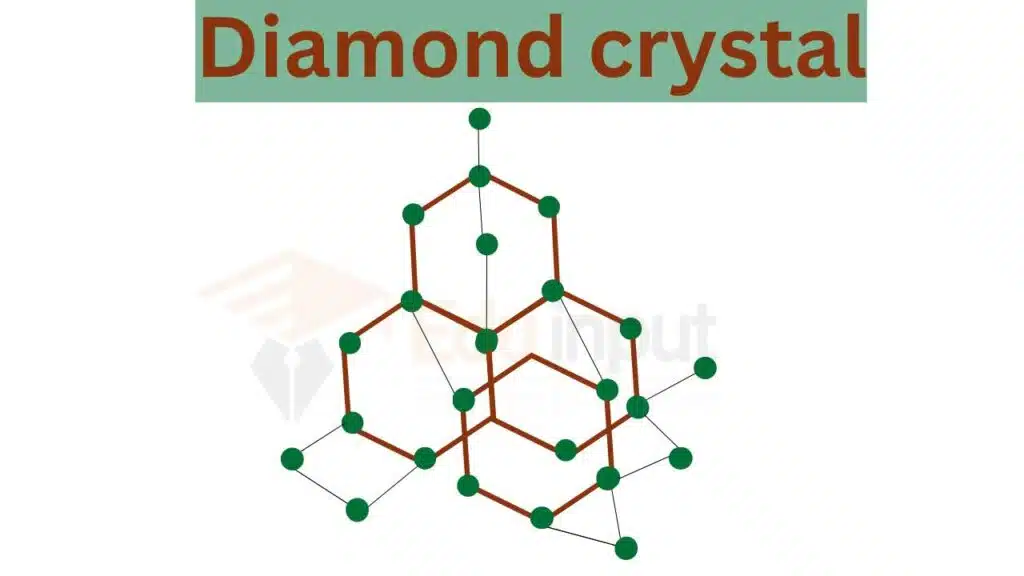
The lattice points in the diamond crystal are the positions where the carbon atoms are located. These lattice points form a three-dimensional array of points that define the crystal lattice.
Crystal lattice and lattice point are both essential concepts in crystallography. The crystal lattice refers to the three-dimensional arrangement of atoms or molecules in a crystal, while the lattice point refers to the positions where the atoms or molecules are located. Understanding these concepts is crucial in studying the properties and behavior of crystalline materials.




Leave a Reply