Dipole moment, definition, types, factors, measurement, properties and applications
Definition
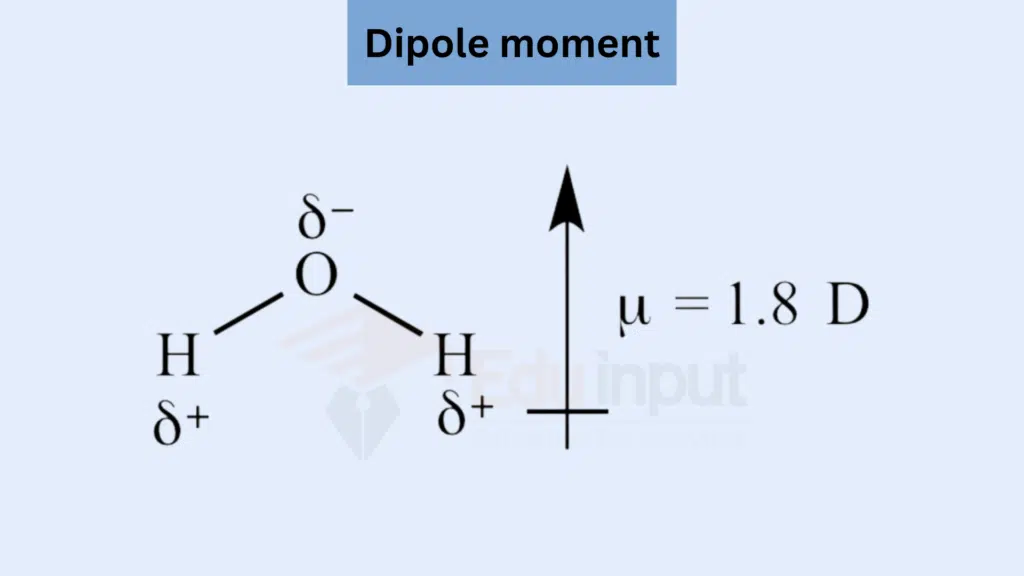
The product of the magnitude of the charge positive or negative and the distance between them is called dipole moment.
Mathematically,
u = q x r
where u (mu) = dipole moment
q = charge
r = distance between the two positive and negative centers
Introduction
In the realm of molecular properties, the dipole moment stands out as a crucial parameter that influences various aspects of a molecule’s behavior. This article aims to provide an in-depth understanding of dipole moments, their representation, units, significance, types, influencing factors, measurement methods, properties of molecules with dipole moments, and their diverse applications.
What is a Dipole Moment?
A dipole moment is a measure of the separation of positive and negative charges within a molecule. It arises when there is an electronegativity difference between atoms in a covalent bond, creating a polar bond with a positive and a negative end. The dipole moment quantifies the overall polarity of a molecule.
The dipole moment is a multifaceted concept that influences the properties and behavior of molecules across various disciplines. Its thorough comprehension is essential for a comprehensive understanding of molecular structures and their diverse applications.
How is a Dipole Moment Represented?
The dipole moment is represented by a vector, pointing from the positive to the negative end of the molecule. The arrowhead indicates the direction of the electron flow.
What are the Units of Dipole Moment?
The standard unit for dipole moment is the debye (D), where 1 debye is equal to 3.336×10−303.336×10−30 coulomb-meter (C·m).
Why is Dipole Moment Important?
The dipole moment is crucial as it provides insights into the charge distribution within a molecule, influencing its physical and chemical properties. It affects intermolecular forces, solubility, and reactivity in various chemical reactions.
Types of Dipole Moments
Permanent Dipole Moments
Arise from permanent charge differences in a molecule due to differences in electronegativity between atoms. Water (H₂O) is an example with a permanent dipole moment.
Induced Dipole Moments
Occur when an external electric field induces a temporary dipole in a molecule. This is a common phenomenon in nonpolar molecules.
Temporary Dipole Moments
Result from instantaneous fluctuations in electron distribution, leading to temporary imbalances in charge. This is observed in all molecules, even those without a permanent dipole.
Factors that Affect Dipole Moment
Electronegativity
The difference in electronegativity between atoms in a bond strongly influences the dipole moment. Greater electronegativity differences lead to stronger dipole moments.
Bond Length
Longer bond lengths tend to have weaker dipole moments compared to shorter bonds. The distance between charges affects the overall dipole moment.
Molecular Geometry
The spatial arrangement of atoms in a molecule plays a significant role. Asymmetrical molecules tend to have larger dipole moments than symmetrical ones.
Measurement of Dipole Moment
Experimental Methods
Techniques like microwave spectroscopy and infrared spectroscopy are commonly used to experimentally determine dipole moments.
Theoretical Methods
Quantum chemical calculations and computational methods are employed to predict dipole moments theoretically.
Properties of Molecules with Dipole Moments
Polarity
Molecules with a nonzero dipole moment are polar, while those with a zero dipole moment are nonpolar.
Intermolecular Forces
Dipole-dipole interactions and hydrogen bonding are stronger in polar molecules, influencing their physical properties.
Solubility
Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents.
Melting and Boiling Points
Generally, polar molecules have higher melting and boiling points due to stronger intermolecular forces.
Electrical Conductivity
Solutions containing polar molecules can conduct electricity to some extent due to the presence of mobile charged particles.
Applications of Dipole Moment
Molecular Structure Determination
Dipole moments aid in determining the three-dimensional geometry of molecules, providing valuable information for structural elucidation.
Material Science
Understanding dipole moments is crucial in designing materials with specific properties, such as polymers and liquid crystals.
Chemistry
Dipole moments play a key role in predicting reactivity, selectivity, and the nature of chemical reactions.
Biology
In biological molecules, dipole moments influence interactions between molecules, such as in the folding of proteins and the structure of membranes.
What is a dipole moment, and how is it defined in the context of molecular chemistry?
A dipole moment is a measure of the separation of positive and negative charges within a molecule, indicating the molecule’s overall polarity. It arises due to differences in electronegativity between atoms in a covalent bond.
How is a dipole moment represented, and what does the direction of the vector signify?
A dipole moment is represented by a vector, pointing from the positive to the negative end of the molecule. The direction of the vector indicates the flow of electrons, from the less electronegative to the more electronegative atom.
What are the units of dipole moment, and why is the debye (D) commonly used?
The standard unit for dipole moment is the debye (D), where 1 debye is equal to 3.336×10−303.336×10−30 coulomb-meter (C·m). The debye is convenient for expressing molecular-scale dipole moments.
Why is dipole moment important in understanding the behavior of molecules?
Dipole moment provides crucial insights into the charge distribution within a molecule, influencing its physical and chemical properties. It affects intermolecular forces, solubility, and reactivity in various chemical reactions.
What are the different types of dipole moments, and how do they differ?
There are three types of dipole moments: permanent dipole moments, induced dipole moments, and temporary dipole moments. Permanent dipole moments arise from permanent charge differences, induced dipole moments result from external electric fields, and temporary dipole moments occur due to instantaneous fluctuations in electron distribution.
What factors affect the magnitude of a molecule’s dipole moment?
The magnitude of a molecule’s dipole moment is influenced by factors such as electronegativity differences between atoms, bond length, and molecular geometry. Greater electronegativity differences and longer bond lengths generally lead to larger dipole moments.





Leave a Reply