DISTRIBUTION OF ELECTRONS IN PERIODS AND GROUPS OF THE PERIODIC TABLE
It is very important to know about the configuration of electrons in the periodic table. In this article, we will discuss electronic distribution in the different periods, groups, and blocks in the modern periodic table.
Period No. 1
There are two elements in this period i.e. hydrogen and helium.

Period No. 2
There are eight elements in this period i.e. from 3Li to 10Ne.

Period No. 3
There are again eight elements in this period i.e. from 11Na to 18A

In order to understand the nature of chemical bonding, it is better that we should learn about the electronic distribution group-wise. Let us do the distribution of I- A and II- A groups.
Group-wise Electronic Distribution:
I- A

I- A group element have one electron in their outermost orbital, that is in s- orbit By moving the electron they develop the unite positive charge and complete the electronic configuration of inert gas. These are called alkali metals. They are s- block elements
II- A

II- A group elements have two electrons in their outermost s- orbital and by losing these two electrons they develop two positive charges. So valency of these elements is two. Alkali and alkaline earth elements are called s- block elements.
III- B

There are three electrons in the outermost principal quantum number and losing and sharing three electrons, the valency of these elements is three since the outermost electron is in p subshells, so they are called p block elements.
IV- A

IV- A group changes show the oxidation state of + 4 and are p- block elements. Their outermost p- shells are half-filled.
V- A

The sub-shells of V- A group elements are half-filled. They show two types of oxidation states (- 3) and (+5). The elements of groups in III-A to VII- A and VIII group elements have their electrons in sub-shells and they are called p- block elements.
By combining p and s, the outermost shell has 5 electrons.
VI- A

The elements VI- A has six electrons in the outermost principal quantum number. The outermost sub-shell is P. They are absorbed p- block elements.
VII- A

VIII (0)

The elements of VIII (A) are inert. They do not have any unpaired electrons.
Electronic Distribution of Transition Elements
4th Period

These ten elements from 21Sc to 30Zn have their d- orbital as outermost and so they are called d- block elements.
Similarly, second transition series;

18 elements are in the 4th period. From which 10 elements belong to d- block, whose outermost shell is 3d
There are three complete series of such elements whose outermost sub-shell is ‘ d ’. The fourth series in period seven is incomplete.
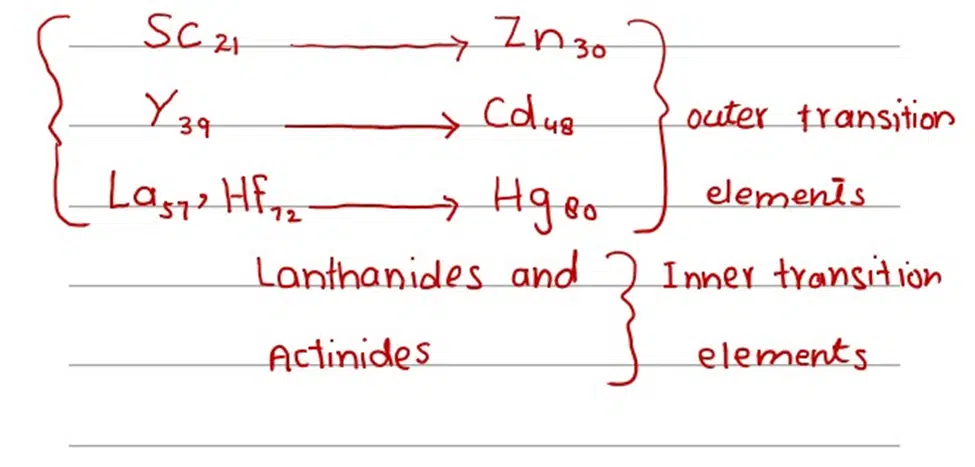
(i) 21Sc —> 30Zn (3d – series)
(ii) 39Y —-> 48Cd (4d – series)
(iii) 57La, 72Hf —> 80Hg (5d – series)
Block elements
There are two series;
(i) 58Ce —> 71Lu (4f – series)
(ii) 89Ac —> 103Lr (5f – series)
These d and f- block elements are called transition elements. d- block elements are called outer transition elements, while f- block elements are called inner transition elements.
Summary
s- Block IA – IIA
p- Block IIIA – VIII(0)
d- Block




Leave a Reply