9 Examples of Alkyl Group
Methyll (H3C-), ethyll (H3C-CH-), propyll (H3C–CH2-CH2-), butyll (H3C–CH2-CH2-CH2-), pentyll (H3C–CH2-CH2-CH2-CH2-), hexyll (H3C–CH2-CH2-CH2-CH2-CH2-), heptyll (H3C–CH2-CH2-CH2-CH2-CH2-CH2-), octyll (H3C–CH2-CH2-CH2-CH2-CH2-CH2-CH2-) and nonyll (H3C–CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-) are some examples of alkyl groups.
These alkyl groups shows carbon backbones ranging from 1-9 atoms, derived by removing hydrogens from parent alkanes.
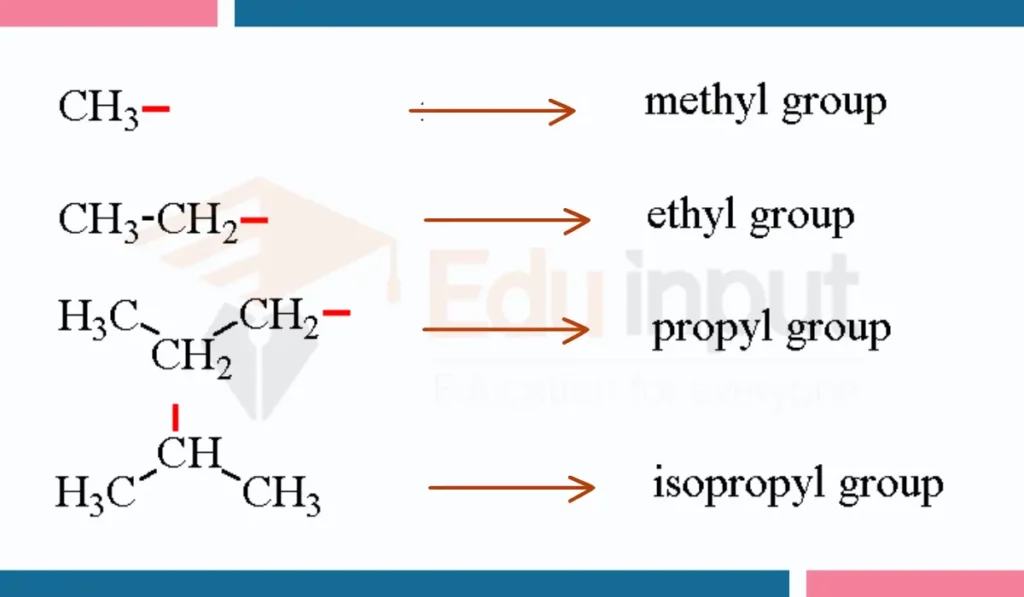
Examples of Alkyl Group
Here are a few Examples of Alkyl Group:
1. Methyll group
The methyll group has the structure H3C-. It consists of a methyl group with one hydrogen atom missing, leaving an open valence through which it can attach to other atoms. The methyll group contains one carbon atom bonded to 3 hydrogen atoms. Since it is derived from methane (CH4) by removing one hydrogen atom, it is considered the simplest alkyl group.
2. Ethyll group
The ethyll group has the structure H3C-CH2-. It consists of an ethyl group missing one hydrogen atom. The ethyll group contains two carbon atoms with one bonded to the other two hydrogen atoms and the other carbon atom missing a hydrogen, leaving it free to bond elsewhere. Since the ethyll group has its origin from ethane (C2H6), it is an alkyl group containing two carbon atoms.
3. Propyll group
The propyll group has the structure H3C–CH2-CH2-. It consists of a propyl group missing one hydrogen atom. The propyll functional group contains three carbon atoms, with two bonded to three hydrogens and the third carbon atom missing a hydrogen atom. As it is derived from propane (C3H8) by removing a hydrogen atom, the propyll group is an alkyl group with a three-carbon backbone.
4. Butyll group
The butyll group has the structure H3C–CH2-CH2-CH2-. It consists of a butyl group missing one hydrogen atom. The butyll functional group contains 4 carbon atoms, with 3 bonded to the maximum number of hydrogens, while the 4th carbon atom is missing a hydrogen, leaving it free to bond elsewhere. As it comes from butane (C4H10) by removing a hydrogen, the butyll group is an alkyl group with a 4-carbon backbone.
5. Pentyll group
The pentyll group has the structure H3C–CH2-CH2-CH2-CH2-. It consists of a pentyl group missing one hydrogen atom. This alkyl contains 5 carbon atoms, 4 bonded to the maximum hydrogens, while the 5th carbon atom is missing a hydrogen atom. Since the pentyll group originates from pentane (C5H12) by removing a hydrogen atom, it is an alkyl group with a 5-carbon backbone.
6. Hexyll group
The hexyll group has the structure H3C–CH2-CH2-CH2-CH2-CH2-. It consists of a hexyl group minus one hydrogen atom. This alkyl group contains 6 carbon atoms, with 5 bonded to the maximum number of hydrogens possible, while the 6th carbon atom has an open valence. As the hexyll functional group comes from hexane (C6H14) by removing a hydrogen, it is an example of a 6-carbon backbone alkyl group.
7. Heptyll group
The heptyll group has the structure H3C–CH2-CH2-CH2-CH2-CH2-CH2-. It consists of a heptyl group missing one hydrogen atom. This alkyl group contains 7 carbon atoms, with 6 bonded to the maximum number of hydrogens and the 7th carbon missing a hydrogen atom. As it originates from heptane (C7H16) by removing a hydrogen atom, the heptyll group is an alkyl functional group with a 7-carbon backbone.
8. Octyll group
The octyll group has the structure H3C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-. It consists of an octyl group minus one hydrogen atom. This alkyl contains 8 carbon atoms, with 7 bonded to the maximum hydrogens possible and the 8th carbon atom missing one hydrogen atom. Since it comes from octane (C8H18) by removing a hydrogen atom, the octyll group is an example of an 8-carbon backbone alkyl group.
9. Nonyll group
The nonyll group has the structure H3C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-. It consists of a nonyl group lacking one hydrogen atom. This alkyl functional group contains 9 carbon atoms. 8 are bonded to the maximum number of hydrogens, while the 9th carbon atom has an open valence where a hydrogen is missing. As the nonyll group is derived from nonane (C9H20) by removing a hydrogen atom, it is an alkyl group with a 9-carbon backbone.





Leave a Reply