15 Examples of Simple Distillation
Simple distillation is a widely used separation technique that exploits differences in boiling points to separate components of a mixture. It is particularly effective for separating volatile liquids from non-volatile impurities. In this article, we’ll explore 15 common examples of simple distillation.
Examples of Simple Distillation
Here are 15 Examples of Simple Distillation:
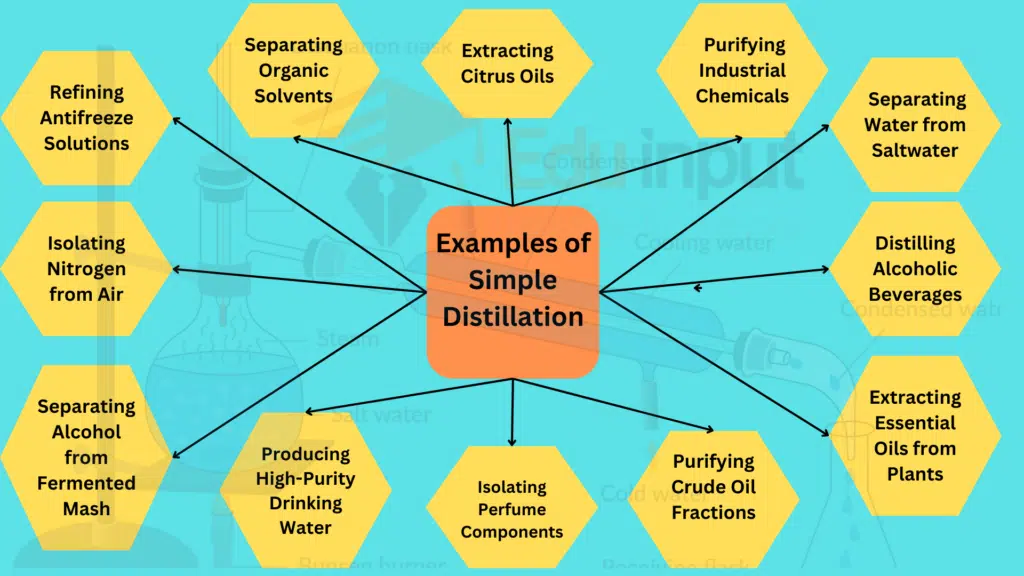
1. Separating Water from Saltwater
It is very common example of simple distillation. Simple distillation is often employed to separate water from saltwater. As the mixture is heated, water vapor rises and is condensed back into liquid form, leaving behind the salt.
2. Isolating Pure Water from a Solution
In laboratories, simple distillation is used to isolate pure water from a solution. By heating the solution, water evaporates, and the vapor is condensed into a separate container, leaving impurities behind.
3. Recovering Solvents from Reaction Mixtures
In chemical processes, simple distillation is used to recover solvents from reaction mixtures. The solvent is separated from other components based on differences in boiling points.
4. Distilling Alcoholic Beverages
The production of alcoholic beverages involves simple distillation. This process separates the alcohol from the fermented mixture, concentrating it into a more potent form.
5. Extracting Essential Oils from Plants
Simple distillation is employed in the extraction of essential oils from plants. The volatile oils vaporize and are condensed, resulting in a concentrated extract.
6. Purifying Crude Oil Fractions
In the petroleum industry, simple distillation is used to purify crude oil fractions. It separates different hydrocarbons based on their boiling points, yielding products like gasoline and diesel.
7. Isolating Perfume Components
The fragrance industry uses simple distillation to isolate components of perfumes. By heating a mixture of aromatic compounds, the desired scents are separated and collected.
8. Producing High-Purity Drinking Water
Simple distillation plays a role in producing high-purity drinking water. By distilling water, impurities are left behind, resulting in a purified and safe drinking water source.
9. Separating Alcohol from Fermented Mash
In the production of alcoholic beverages, simple distillation separates alcohol from the fermented mash. This concentration of alcohol is essential for various types of spirits.
10. Isolating Nitrogen from Air
In industrial settings, simple distillation is used to isolate nitrogen from air. The different boiling points of nitrogen and other components in air allow for their separation.
11. Refining Antifreeze Solutions
The production of antifreeze solutions involves simple distillation to refine the mixture. This process removes impurities and concentrates the active ingredients.
12. Separating Organic Solvents
Laboratories utilize simple distillation to separate organic solvents. This technique is crucial for ensuring the purity of solvents used in various chemical processes.
13. Extracting Citrus Oils
Citrus oils, used in the food and fragrance industries, are extracted using simple distillation. The process separates the volatile oils from citrus peels.
14. Purifying Industrial Chemicals
In chemical manufacturing, simple distillation is employed to purify industrial chemicals. This ensures that the final product meets specific quality standards.
15. Concentrating Acids in Laboratories
Simple distillation is used in laboratories to concentrate acids. By carefully controlling the distillation process, the acid is separated from water or other components.



Leave a Reply