Molecular Weight and Diffusion Rate-How it Affects Diffusion
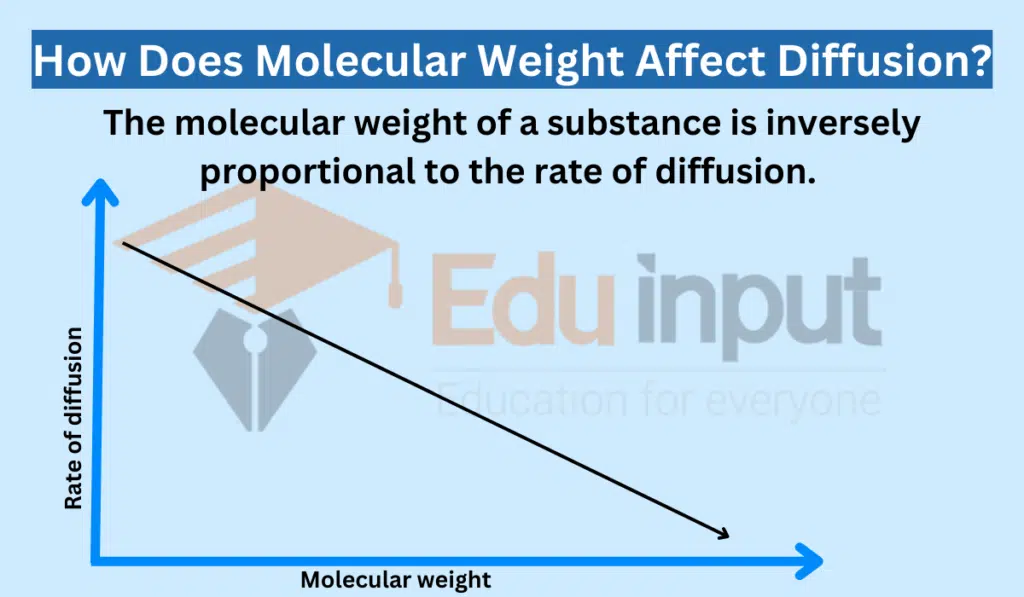
The molecular weight of a substance is inversely proportional to the rate of diffusion. This means that heavier molecules diffuse more slowly than lighter molecules.
Also learn Why Does Diffusion Occurs?
Effect of Molecular Weight on Diffusion
The rate of diffusion decreases as molecular weight increases. There are a few reasons for this.
First, heavier molecules have more inertia, which means they are more resistant to changes in motion.
Second, heavier molecules have more collisions with other molecules, which slows them down.
Finally, heavier molecules tend to be more attracted to each other, which also slows down their diffusion.
For example, oxygen (O2) has a molecular weight of 32, while carbon dioxide (CO2) has a molecular weight of 44. This means that CO2 diffuses more slowly than O2.
| Substance | Molecular Weight | Diffusion Coefficient (cm^2/s) |
|---|---|---|
| Oxygen | 32 | 1.82 x 10^-5 |
| Carbon dioxide | 44 | 1.53 x 10^-5 |
| Ammonia | 17 | 2.21 x 10^-5 |
| Water | 18 | 2.3 x 10^-5 |
As you can see, the diffusion coefficient decreases as the molecular weight increases. This is because heavier molecules have more inertia and are more likely to collide with other molecules, which slows down their diffusion.
The effect of molecular weight on diffusion is important in many different fields, including biology, chemistry, and physics.
For example, in biology, the diffusion of oxygen and carbon dioxide is essential for cell respiration. In chemistry, the diffusion of molecules is used in a variety of separation techniques, such as chromatography.
And in physics, the diffusion of neutrons is used to study the properties of materials.






Leave a Reply