Neodymium-Discovery, Properties, And Applications
Neodymium is a chemical element with the symbol Nd and atomic number 60. It belongs to the rare earth elements group and is the third most abundant of the rare earth elements after cerium and lanthanum. Neodymium is a soft, silvery metal that tarnishes in air. It is known for its magnetic properties and is used in the production of strong permanent magnets.
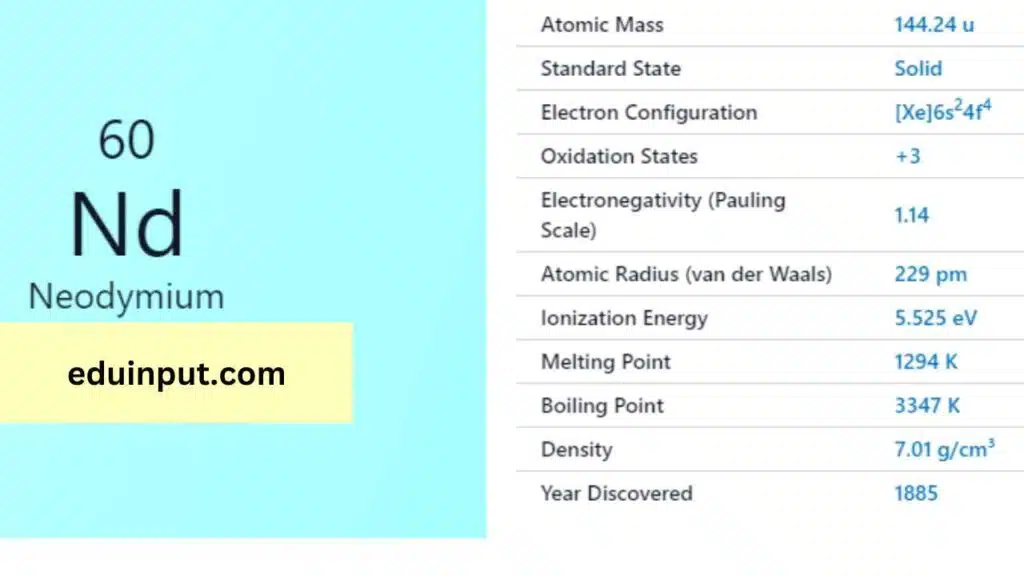
| Property | Value |
| Name | Neodymium |
| Symbol | Nd |
| Atomic number | 60 |
| Relative atomic mass (Ar) | The group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white, yellowish tinge |
| Classification | Metallic |
| The group in the periodic table | |
| Period in the periodic table | Lanthanoid |
| Block in the periodic table | 6 (lanthanoid) |
| The group in periodic table | f |
| Shell structure | 2.8.18.22.8.2 |
| CAS Registry | 7440-00-8 |
Discovery
Neodymium was first discovered in 1885 by Austrian chemist Carl Auer von Welsbach, who separated it from didymium, a mixture of rare earth elements.
Physical Properties
Neodymium is a silvery-white metal that is malleable and ductile. It has a melting point of 1,021°C and a boiling point of 3,074°C. Neodymium has a density of 7.01 g/cm³, which is about one-third lighter than iron. It is also highly reactive and easily oxidizes when exposed to air.
Chemical Properties
Neodymium is a rare earth element and is classified as a metal. It is highly reactive and reacts with most nonmetals and metals, including oxygen, nitrogen, and water. It forms various compounds, including oxides, halides, and sulfates.
Facts
Neodymium is used to make high-strength permanent magnets for various applications, such as in computer hard drives, electric motors, and headphones. It is also used in the production of colored glass, lasers, and in lighting. Neodymium is also used in nuclear reactors as a dopant for enhancing nuclear reactions.
Applications
Neodymium is widely used in the production of high-strength permanent magnets, which are used in many modern applications, including wind turbines, hybrid cars, and electric motors. It is also used in the production of glass for lighting and for making color filters for televisions and computer monitors. Neodymium is also used in the manufacture of laser materials, ceramic capacitors, and in nuclear reactors.





Leave a Reply