Nobelium-Discovery, Properties, And Applications
Nobelium is a synthetic, radioactive element with the symbol No and atomic number 102. It is a member of the actinide series of elements and was first synthesized in 1957.
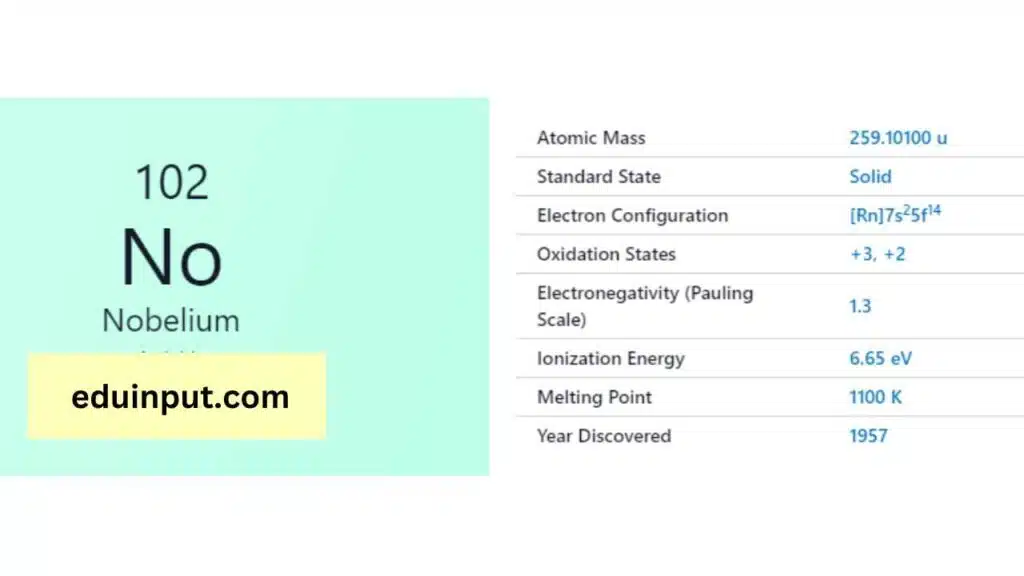
| Property | Value |
| Name | Nobelium |
| Symbol | No |
| Atomic number | 102 |
| Relative atomic mass (Ar) | (longest-lived isotope) |
| Standard state | Presumably a solid at 298 K |
| Appearance | Unknown, but probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Actinoid |
| Period in periodic table | 7 (actinoid) |
| Block in periodic table | f |
| Shell structure | 2.8.18.32.32.8.2 |
| CAS Registry | 10028-14-5 |
Discovery
Nobelium was first synthesized in 1957 by a team of scientists led by Albert Ghiorso at the Lawrence Berkeley National Laboratory in California. The element was named after Alfred Nobel, the inventor of dynamite and founder of the Nobel Prizes.
Physical Properties
Nobelium is a silvery-white metal that is highly radioactive. It is the heaviest element of the actinide series and has a density of 9.9 g/cm3.
Chemical Properties
Nobelium is a highly reactive element and has not been found in the Earth’s crust. It is difficult to study its chemical properties due to its short half-life and small quantities produced in laboratories.
Facts
- Nobelium has no stable isotopes
- The most stable isotope, nobelium-259, has a half-life of just 58 minutes
- Nobelium has no known biological role
- The element has not been found naturally occurring on Earth and is only produced in laboratories through nuclear reactions
Applications
Nobelium has no practical applications due to its instability and limited availability. However, its study is important in advancing our understanding of the properties of heavy elements and the principles of nuclear physics.
Nobelium is a highly reactive and radioactive element that was first synthesized in 1957. It has no known biological role and has no practical applications due to its instability and limited availability. Its study is important in advancing our understanding of heavy elements and nuclear physics.





Leave a Reply