Planck’s quantum theory-History, evidence and applications
History of Planck’s Quantum Theory
By the end of the 19th century, scientists were able to explain most natural phenomena by using classical theory. Matter and energy used to be separate from one another. James Clerk Maxwell, a Scottish physicist, gave the equations used to define the properties of radiant energy in 1873.
The photoelectric effect can’t be explained by classical theory or classical mechanics according to scientists by the 20th century. The German physicist Max Planck came up with a theory of the quantized nature of the energy of waves.
Evidence for a particle theory of energy, radiation, black body radiation, and quantum theory of radiation are some of the topics discussed in this article.
What is Planck’s Quantum Theory?
In 1900, Max Planck gave his theory about light. This theory is called Plank’s quantum theory.
Postulates of Planck’s quantum theory
This theory has three postulates.
- Light is not emitted or absorbed continuously
- Light is emitted or absorbed in the form of packets of energy. These packets of energies are called photons or quanta.
- The energy associated with a photon is given by the formula.
E = hv
‘E’ is the energy of the photon is ergs or joules. ‘v’ is the frequency of the photon.
Frequency of photon
The frequency of the photon (v) is the number of waves produced per second.
All the photons of light have the same velocity so their energies depend upon their frequency is measured in wave/sec, cycle/sec, or S-1 or Hertz (1 cycle/sec =1 Hz)
‘h’ is Planck’s constant. It is the ratio of the energy of a photon to the frequency of that photon. The units of ‘h’ are as follows:
E = hv
When energy is taken in ergs
H = E/v = erg/sec-1 = erg.sec
When the energy of a photon is taken in joules
Then h = E/v = joule/sec-1 = J.sec
The value of the energies and the frequencies of photons are adjusted by nature. In such a way the ratio of these two quantities is always constant. Its value is:
h = 6.625 x 10-27 erg.sec
h = 6.625 x 10 -34 J.sec
1J = 10 +7 ergs
Wavelength of photon
The wavelength of a photon is the distance between two adjacent crests or troughs.
It has units of length i.e. meter or centimeter. Since the wavelength of a photon is very very small, so it is expressed in Ao, nm, or pm.
1Ao = 10-10m = 10-8cm
1nm = 10-9m = 10-7cm
1p.m = 10-12m = 10-10cm
The wavelength and frequency of a photon are inversely proportional to each other.
v = c/λ —> (2)
Put equation (2) in equation (1)
E = hc/ λ
So, the greater the wavelength of a photon, the smaller the energy associated with a photon.
Wave number of the photon
The wave number of the photon is the number of waves per unit distance cm-1 or m-1.
It is just the inverse of wavelength. It is represented by V Bar.
v Bar = 1/ λ
Put equation (4) in equation (2)
V = cv Bar
So, the greater the wave number of a photon, the greater the frequency.
Put equation (5) in (1)
E = hc v Bar
Hence, the greater the wave number of a photon, the greater the energy associated with the photon.
Evidence in Support of Planck’s Quantum Theory
There were many experiments that were performed to analyze the quantum theory. Strong shreds of evidence for quantum theory were supported by all experimental observations. The quantized energy of the electron motion in the matter is shown in all of them.
The light can be different depending on the wavelength of the prism. If the light only behaves as a wave, then the rainbow should be continuous. This also supports the theory of quantum mechanics. The quantum theory of radiation is supported by the emission spectrum of nitrogen gas.
Applications of Planck’s Quantum Theory
The fundamental theory of quantum mechanics is Planck’s quantum theory. There are applications for quantum mechanics in a lot of fields. It is used in electrical appliances, the medical field, quantum computing, lasers, and quantum cryptography.
Also Read: Limitations of Bohr’s Atomic Model

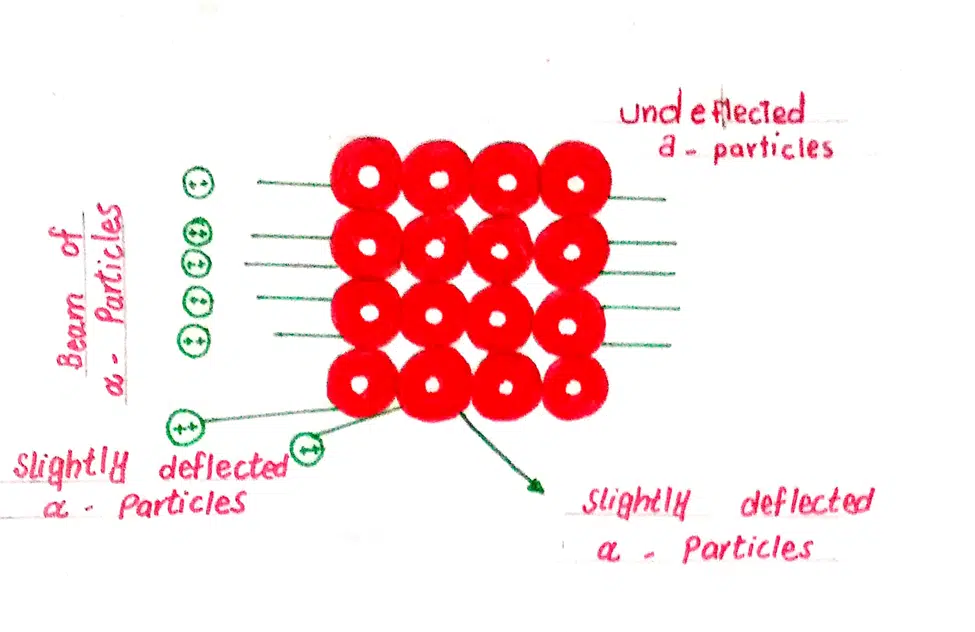
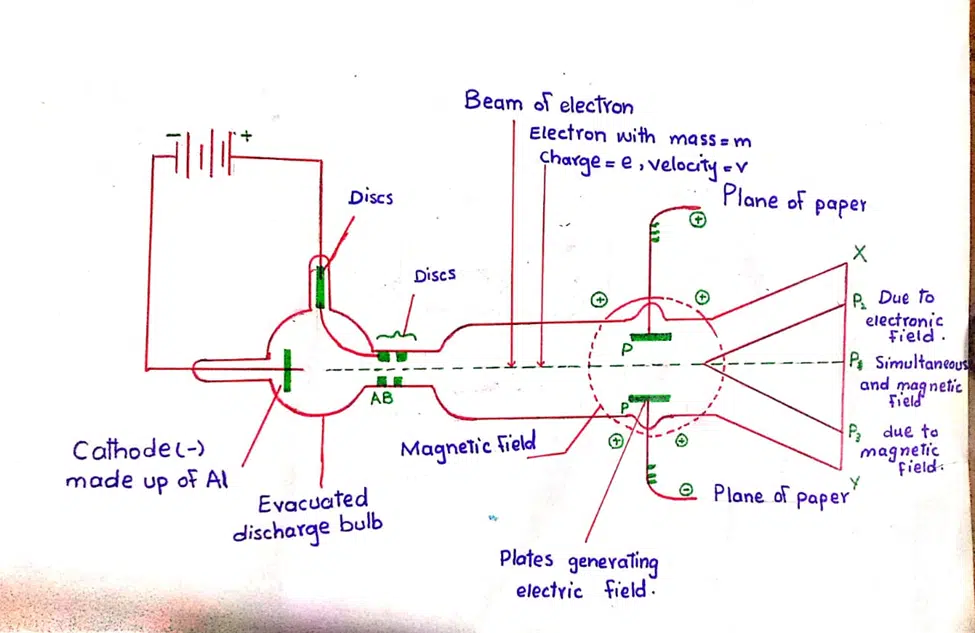
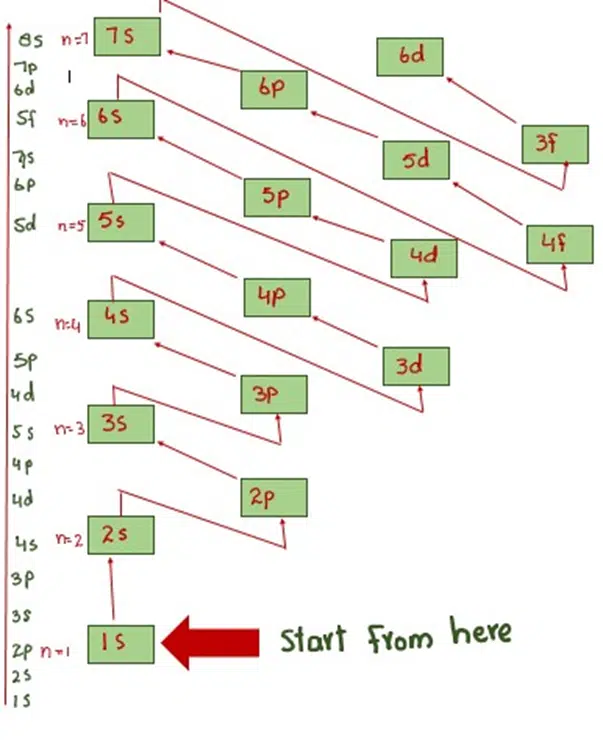


Leave a Reply