Rutherfordium-Properties And Applications
Rutherfordium is a synthetic chemical element with the symbol ‘Rf’ and atomic number 104. It is a highly radioactive metal that was first synthesized in 1964 by a team of scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. Rutherfordium is named after the physicist Ernest Rutherford, who made significant contributions to the study of atomic structure and nuclear physics.
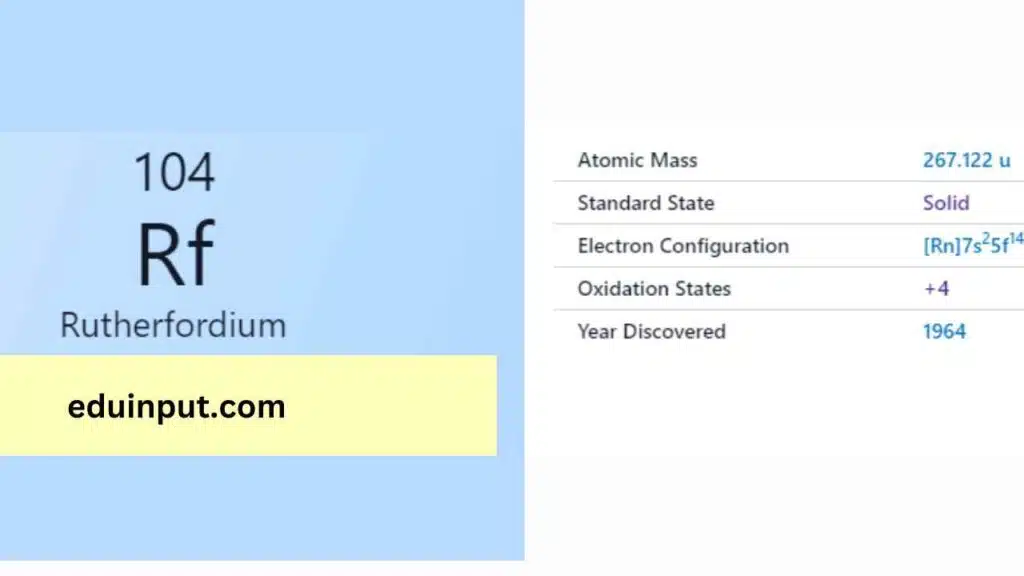
| Property | Value |
| Name | Rutherfordium |
| Symbol | Rf |
| Atomic number | 104 |
| Relative atomic mass (Ar) | (longest-lived isotope) |
| Standard state | Presumably a solid at 298 K |
| Appearance | Unknown, probably metallic and silvery white or grey in appearance |
| Classification | Metallic |
| Group in periodic table | 4 |
| Group name | (none) |
| Block in the periodic table | 7 |
| Group in the periodic table | d |
| Shell structure | 2.8.18.32.32.10.2 |
| CAS Registry | 53850-36-5 |
Physical Properties
As a synthetic element, very little is known about the physical properties of rutherfordium. It is believed to be a dense, silvery metal with a high melting point and boiling point. Rutherfordium is highly radioactive, and its isotopes have short half-lives, making it difficult to study.
Chemical Properties
Like other elements in the transition metal series, rutherfordium is expected to have a high melting point and boiling point and to form a range of chemical compounds with other elements. Due to its highly radioactive nature, however, very little is known about the chemical properties of rutherfordium.
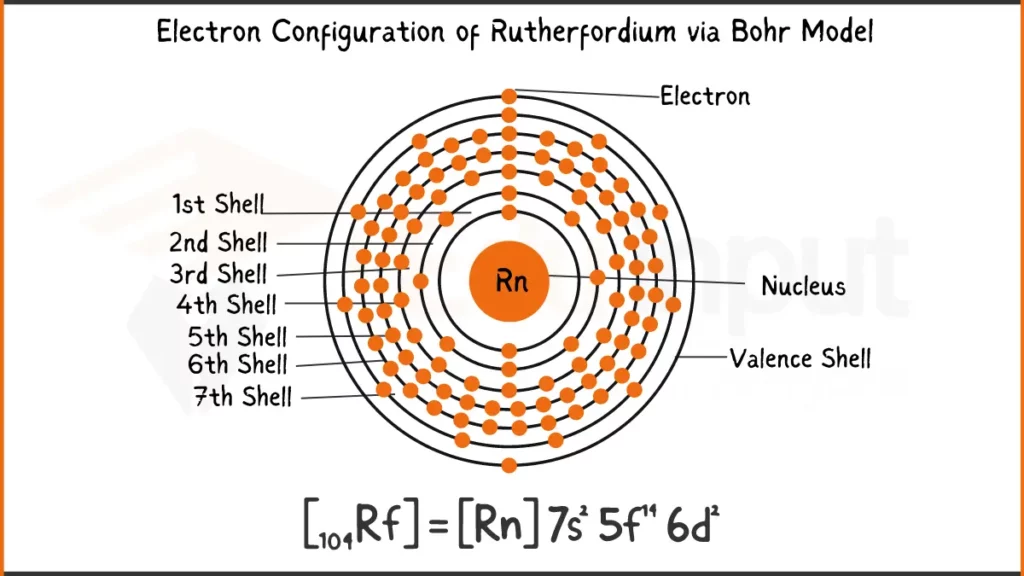
Electronic Configuration of Rutherfordium
Rutherfordium (Rf) packs 104 electrons ([Rn] 5f¹⁴ 6d² 7s². This element stands out with a mere two electrons (6d²) in its outermost shell, influencing its unique bonding behavior.
Electronic Configuration of Rutherfordium via Bohr Model
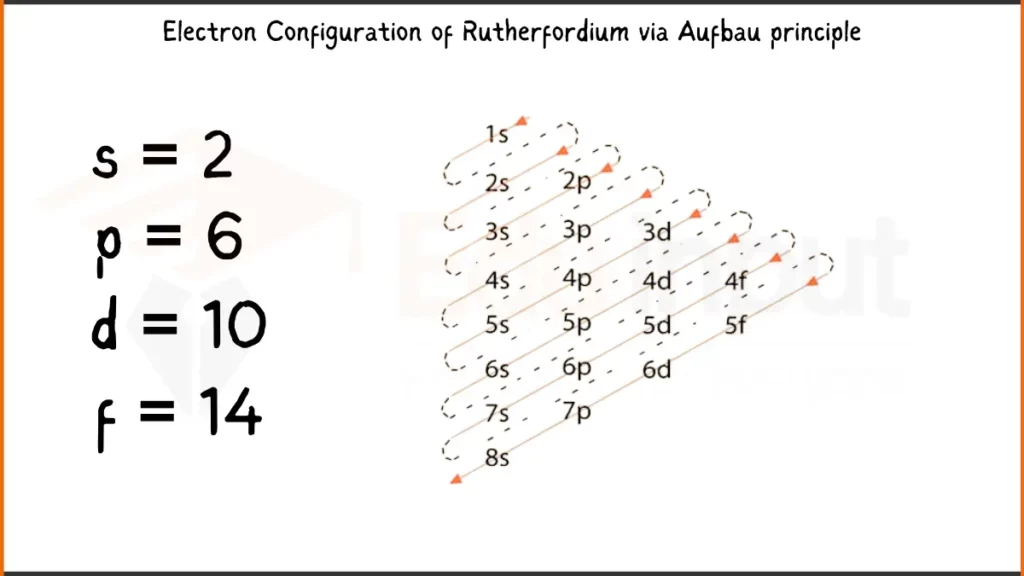
Electronic Configuration of Rutherfordium via Aufbau Principle
Facts
- Rutherfordium is a synthetic element that was first synthesized in 1964 by a team of scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia.
- Rutherfordium is named after the physicist Ernest Rutherford, who made significant contributions to the study of atomic structure and nuclear physics.
- Rutherfordium is highly radioactive, and its isotopes have short half-lives, making it difficult to study.
Applications
Due to its highly radioactive nature, rutherfordium has no practical applications outside of scientific research. Its primary use is in the study of nuclear physics and the properties of heavy elements. Rutherfordium has been used in experiments to study the production of superheavy elements, and its properties have been used to help understand the behavior of other elements in the same group.
Rutherfordium is a synthetic chemical element with fascinating properties that make it an important element for the study of nuclear physics and the properties of heavy elements. Due to its highly radioactive nature, rutherfordium has no practical applications outside of scientific research, but its study is important for advancing our understanding of the universe and the behavior of matter at the atomic and subatomic levels.




Leave a Reply