RUTHERFORD’S ATOMIC MODEL (DISCOVERY OF NUCLEUS)
Introduction to Rutherford’s atomic model
Rutherford’s atomic model is based upon laws of motion and laws of gravitation. His model is based on his experiment on the scattering of alpha particles. He thought that the system of atoms is just like the solar system. The nucleus represents the sun and the revolving electrons represent the planets.
Related article: What is an atomic model in chemistry?
Experiments By Rutherford
Rutherford obtained a narrow beam of an alpha particle from a radioactive substance, radium. He directed this beam at a thin sheet of gold foil of thickness 0.00004 cm. A screen coated with zinc sulfide was placed on the other side of the foil.
He noticed that most of the alpha particles passed straight through the foil without any deflection from their path. A few of them were deflected at some angles more than 90o.
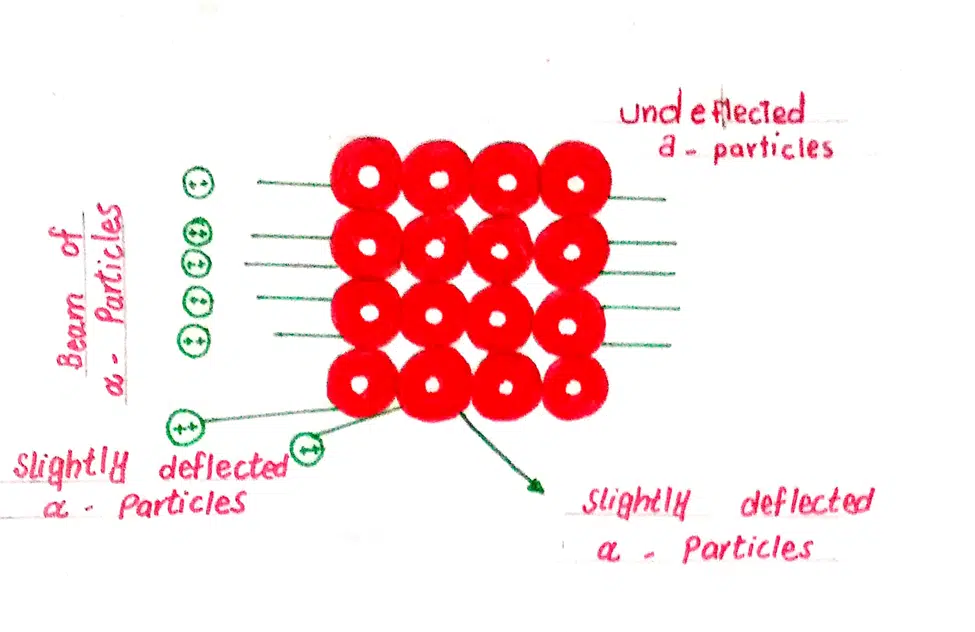
Very few deflected back on the original path on the basis of these observations, he reached the following conclusions.
Findings of Rutherford’s atomic model
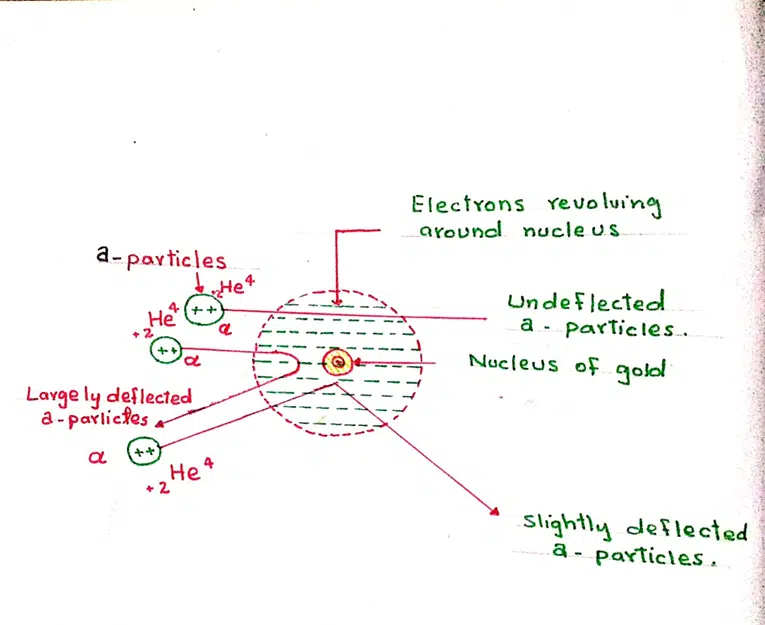
1. Atom contains a heavy and positively charged part at the center. This heavy part of the atom is called its nucleus.
2. The volume occupied by the nucleus is only a minute fraction of the total volume of the atom.
3. All the space outside the nucleus is occupied by the electron. The number of electrons outside the nucleus is equal to the number of protons in the nucleus.
4. The electrons are not stationary. They are in constant motion around the nucleus.
5. Except for electrons all other particles lie within the nucleus.
DEFECTS OF RUTHERFORD’S MODEL
1. Rutherford’s model is based on the laws of motion and gravitation. These laws can be applied to neutral bodies like planets but not to charged bodies such as electrons and protons.
2. The revolving electron being negatively charged should emit the energy continuously. The orbit of the electron should fall smaller and smaller. Due to this spiral path electron should fall into the nucleus but it never falls.
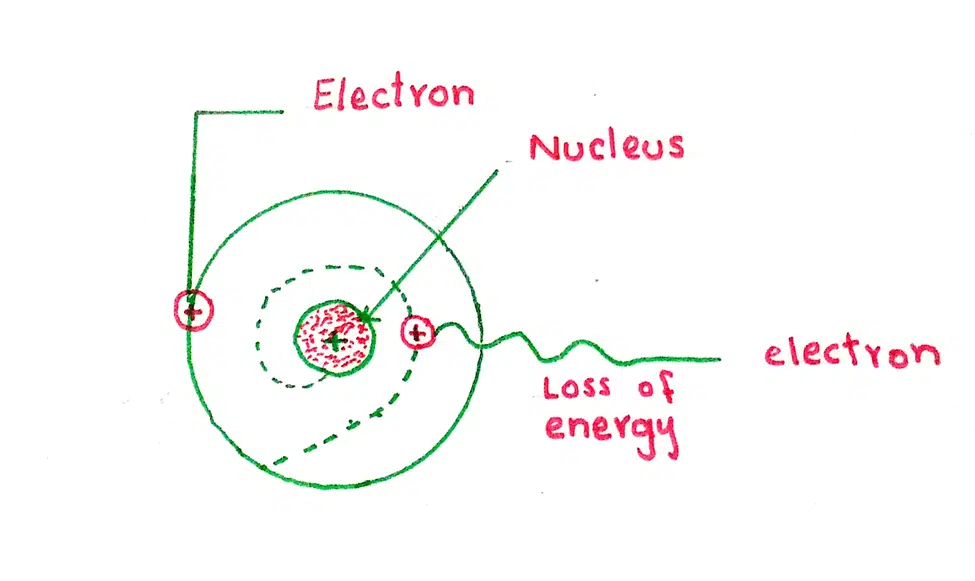
3. If the electron radiates energy continuously, we should get a continuous spectrum. Actually, atoms give a line spectrum.




Leave a Reply