Sp-hybridization, definition, explanation, examples and significance
Definition
The process in which one s and one p orbitals intermix to form two sp-hybrid orbitals is called sp-hybridization.
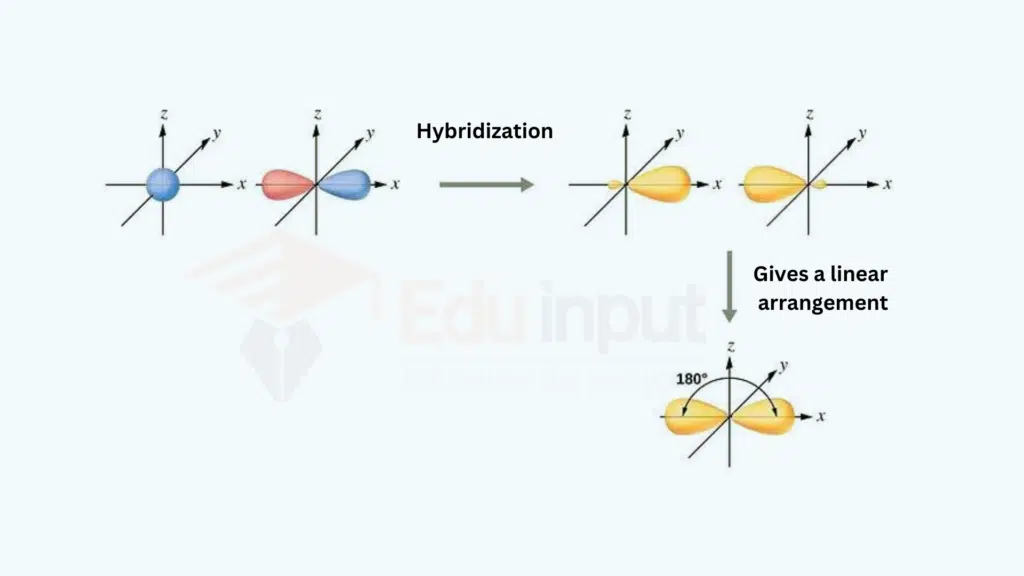
What is sp-hybridization?
In molecular chemistry the concept of sp hybridization stands out as a fundamental and intriguing phenomenon. This hybridization involves the fusion of one s orbital with one p orbital, leading to the formation of two equivalent sp hybrid orbitals. The outcomes of sp hybridization play a pivotal role in shaping molecules with linear geometry, influencing properties and reactivity.
Its role in forming triple bonds, facilitating conjugation, and shaping linear molecular structures underscores its significance in advancing our understanding of the molecular world. The exploration of sp hybridization opens doors to unraveling the intricacies of molecular architecture and paves the way for innovations across various scientific disciplines.
Sp-hybridization Examples
The compounds like BeCl2 and CH ≡CH are the best examples of sp-hybridization.
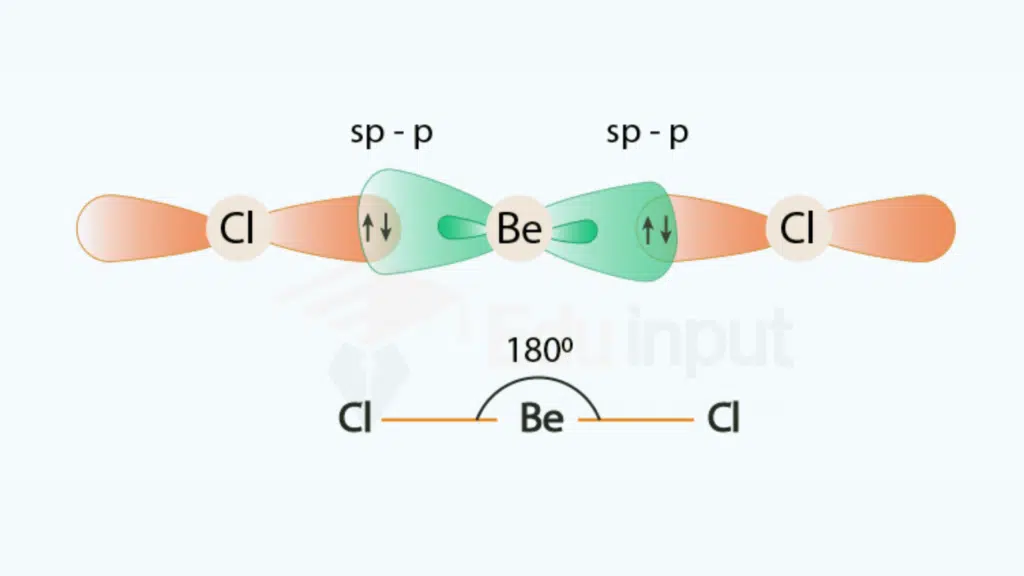
(i) Beryllium chloride, BeCl2
In the electronic configuration of the excited state of Be, one electron from 2s is promoted to 2p orbital. Thus Be becomes divalent. One 2s and one 2p orbitals undergo hybridization to form two sp-hybrid orbitals which are oriented at an angle of 180° showing linear geometry.
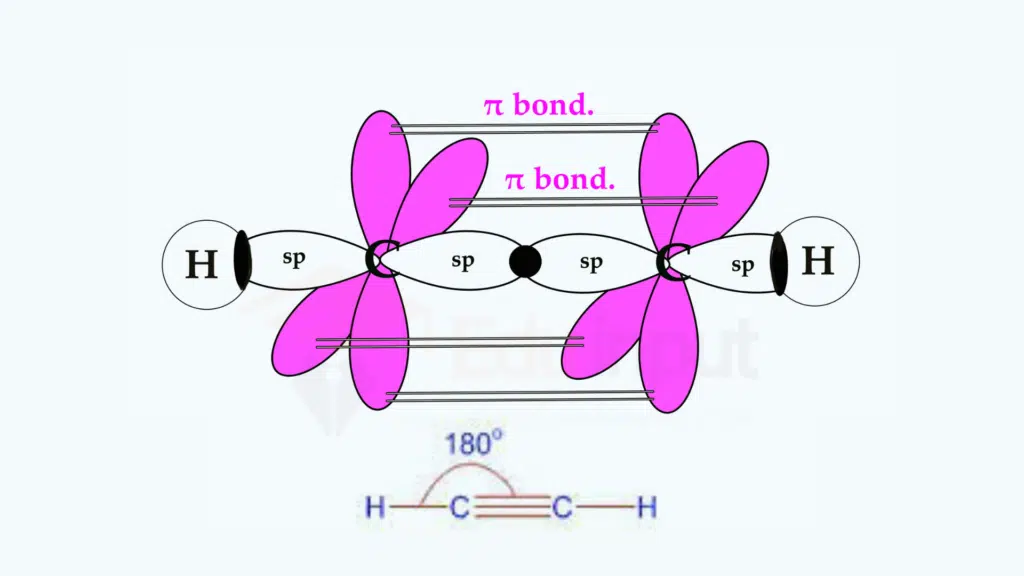
(ii) Ethyne, C2H2
In an ethyne molecule, each carbon atom undergoes sp-hybridization. One 2s and one 2p orbitals mix together to form two sp-hybrid orbitals.
One sp-hybrid orbital undergoes head-to-head overlap with the sp-hybrid orbital of other carbon to form a (C-C) sigma bond. The other sp-hybrid orbital of each carbon overlaps with the 1s orbital of hydrogen atoms forming two sigma bonds. Each carbon atom is left with two hybridized p orbitals i.e., py, and pz, which are parallel to each other in separate planes. These half-filled orbitals of both the carbon atoms undergo sideway overlap to form two π -bonds.
Significance of sp Hybridization in Molecular Chemistry
sp hybridization is a fundamental concept in molecular chemistry that plays a crucial role in determining the structure, geometry, and reactivity of various compounds. The significance of sp hybridization extends across different areas of chemistry and has several key implications:
1. Linear Molecular Geometry
The most apparent significance of sp hybridization is the formation of linear molecular geometry. This is essential in understanding the overall shape of molecules, influencing their physical and chemical properties.
2. Triple Bond Formation
sp hybridization is associated with the formation of triple bonds in molecules. For example, in acetylene (ethyne), carbon atoms undergo sp hybridization, facilitating the creation of one sigma (σ) bond and two pi (π) bonds.
3. Conjugated Systems in Organic Chemistry
Conjugated systems, such as those found in aromatic compounds, benefit from sp hybridization. The unhybridized p orbital contributes to the stability of pi (π) electron delocalization in these systems.
5. Nanomaterials and Nanotechnology
sp hybridization plays a vital role in the formation of various nanomaterials, including carbon nanotubes. The remarkable mechanical, thermal, and electrical properties of these materials find applications in nanotechnology.
6. Organic Synthesis and Catalysis
In organic synthesis, the understanding of sp hybridization guides chemists in planning efficient synthetic routes. Additionally, the linear geometry and reactivity associated with sp hybridization influence catalytic processes.
7. Biological Molecules
Certain biological molecules, including amino acids, peptides, and proteins, exhibit sp hybridization in specific functional groups. This hybridization affects the geometry and reactivity of these molecules.
What is sp hybridization?
sp hybridization is a type of hybridization in chemistry where one s orbital and one p orbital of an atom combine to form two equivalent sp hybrid orbitals. These orbitals have linear geometry and are crucial in the formation of double or triple bonds in molecules.
Which atoms commonly undergo sp hybridization?
Carbon atoms are the most common examples of atoms that undergo sp hybridization. This hybridization occurs in molecules with double or triple bonds, such as alkynes and certain types of organic compounds.
How does sp hybridization lead to the formation of a triple bond?
In sp hybridization, one s orbital and one p orbital combine, resulting in two sp hybrid orbitals. These orbitals are then involved in sigma (σ) bond formation, and the unhybridized p orbitals contribute to the formation of two pi (π) bonds, resulting in a triple bond.
What is the significance of sp hybridization in molecular geometry?
The significance lies in the linear geometry formed by sp hybrid orbitals. This linear arrangement affects the overall shape of molecules, influencing their physical and chemical properties.
How does sp hybridization differ from sp² and sp³ hybridization?
The main difference lies in the number of p orbitals involved in the hybridization. In sp hybridization, one s orbital and one p orbital combine, forming two sp hybrid orbitals. In sp² hybridization, one s orbital and two p orbitals combine, forming three sp² hybrid orbitals. In sp³ hybridization, one s orbital and three p orbitals combine, forming four sp³ hybrid orbitals. The resulting geometry varies from linear (sp) to trigonal planar (sp²) to tetrahedral (sp³).







Leave a Reply