Sulfur-Discovery, Properties, And Applications
Sulfur is a non-metallic chemical element that has the symbol S and atomic number 16. It is abundant in the earth’s crust and is found in minerals such as sulfates and sulfides. Sulfur is a vital element for all living organisms, and it plays an important role in many industrial processes.

| Property | Value |
| Name | Sulfur |
| Symbol | S |
| Atomic number | 16 |
| Relative atomic mass (Ar) | 32.06 range: [32.059, 32.076] |
| Standard state | Solid at 298 K |
| Appearance | Lemon yellow |
| Classification | Non-metallic |
| Period in the periodic table | 16 |
| Group name | Chalcogen |
| Period in periodic table | 3 |
| Block in periodic table | p |
| Shell structure | 2.8.6 |
| CAS Registry | 7704-34-9 |
Discovery
Sulfur has been known since ancient times. The ancient Greeks and Romans used sulfur as a medicine and for bleaching cloth. In 1777, Antoine Lavoisier discovered that sulfur is an element.
Electron Configuration of Sulfur
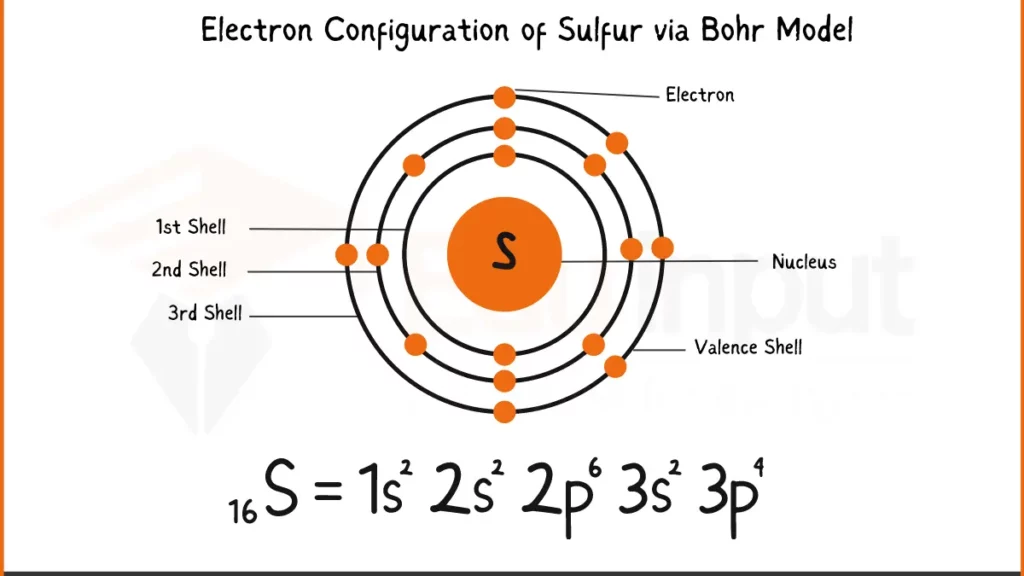
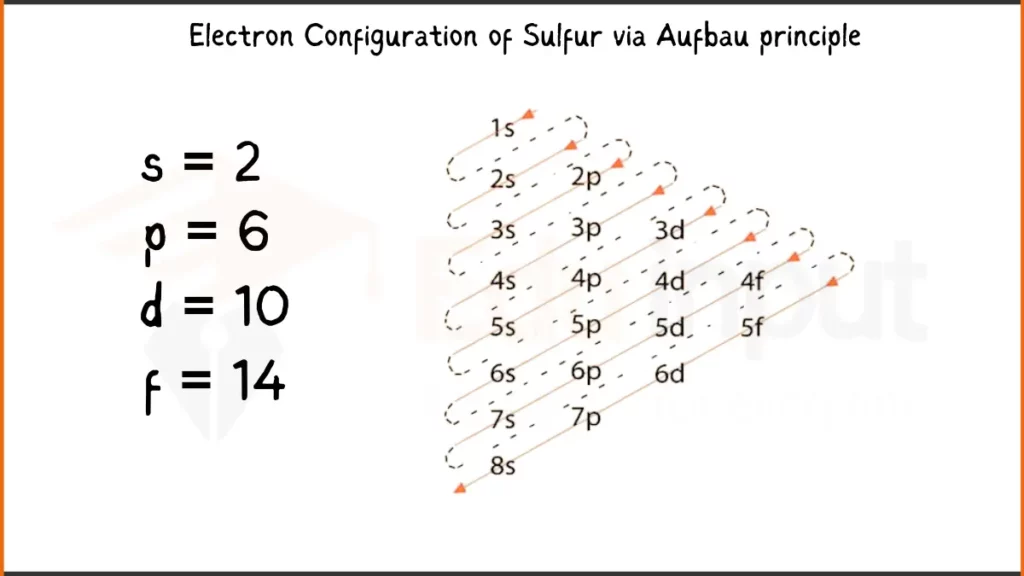
Sulfur (S) packs 16 electrons. Its electronic configuration is 1s²2s²2p⁶3s²3p⁴. This shows to 2 electrons each in the first two shells, followed by 6 in the next, another 2 in the third, and finally 4 in the outermost shell.
Electron Configuration of Sulfur via Bohr Model
Electron Configuration of Sulfur via Aufbau Principle
Physical Properties
Sulfur is a yellow, brittle solid at room temperature. It has a melting point of 115.21°C and a boiling point of 444.6°C. It is insoluble in water but soluble in organic solvents. Sulfur exists in several allotropes, including rhombic, monoclinic, and amorphous sulfur.
Chemical Properties
Sulfur is a highly reactive element that readily forms compounds with other elements. It is a non-metal and is located in group 16 of the periodic table. Sulfur is a powerful reducing agent and can combine with almost all other elements. Sulfur is used in the production of sulfuric acid, which is one of the most important industrial chemicals.
Facts
- Sulfur is a vital element for all living organisms.
- Sulfur is used in the production of sulfuric acid, which is one of the most important industrial chemicals.
- Sulfur is a non-metal and is located in group 16 of the periodic table.
- Sulfur is a highly reactive element that readily forms compounds with other elements.
Applications
Sulfur is used in many industrial processes, including the production of sulfuric acid, fertilizers, and rubber products. It is also used in the production of pesticides, dyes, and pharmaceuticals. Sulfur is used as a preservative for dried fruits and is also used to vulcanize rubber. In addition, sulfur is used in the production of black powder, which is a type of gunpowder.





Leave a Reply